Input interpretation
ammonia | benzene
Chemical names and formulas
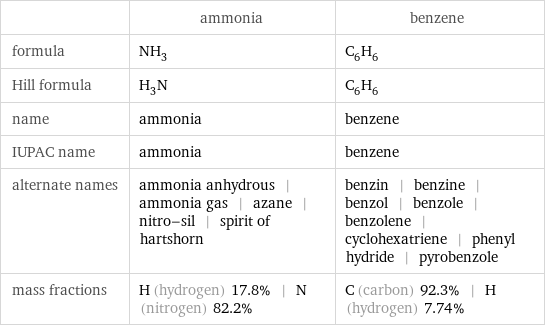
| ammonia | benzene formula | NH_3 | C_6H_6 Hill formula | H_3N | C_6H_6 name | ammonia | benzene IUPAC name | ammonia | benzene alternate names | ammonia anhydrous | ammonia gas | azane | nitro-sil | spirit of hartshorn | benzin | benzine | benzol | benzole | benzolene | cyclohexatriene | phenyl hydride | pyrobenzole mass fractions | H (hydrogen) 17.8% | N (nitrogen) 82.2% | C (carbon) 92.3% | H (hydrogen) 7.74%
Structure diagrams
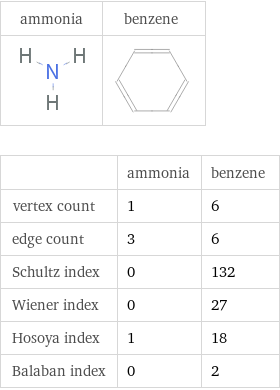
| ammonia | benzene vertex count | 1 | 6 edge count | 3 | 6 Schultz index | 0 | 132 Wiener index | 0 | 27 Hosoya index | 1 | 18 Balaban index | 0 | 2
3D structure
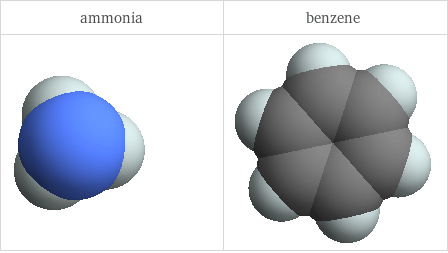
3D structure
Basic properties
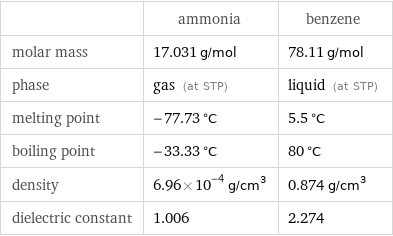
| ammonia | benzene molar mass | 17.031 g/mol | 78.11 g/mol phase | gas (at STP) | liquid (at STP) melting point | -77.73 °C | 5.5 °C boiling point | -33.33 °C | 80 °C density | 6.96×10^-4 g/cm^3 | 0.874 g/cm^3 dielectric constant | 1.006 | 2.274
Gas properties
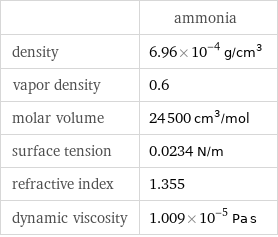
| ammonia density | 6.96×10^-4 g/cm^3 vapor density | 0.6 molar volume | 24500 cm^3/mol surface tension | 0.0234 N/m refractive index | 1.355 dynamic viscosity | 1.009×10^-5 Pa s
Units

Liquid properties
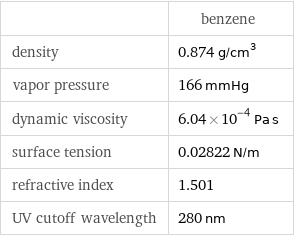
| benzene density | 0.874 g/cm^3 vapor pressure | 166 mmHg dynamic viscosity | 6.04×10^-4 Pa s surface tension | 0.02822 N/m refractive index | 1.501 UV cutoff wavelength | 280 nm
Units

Thermodynamic properties
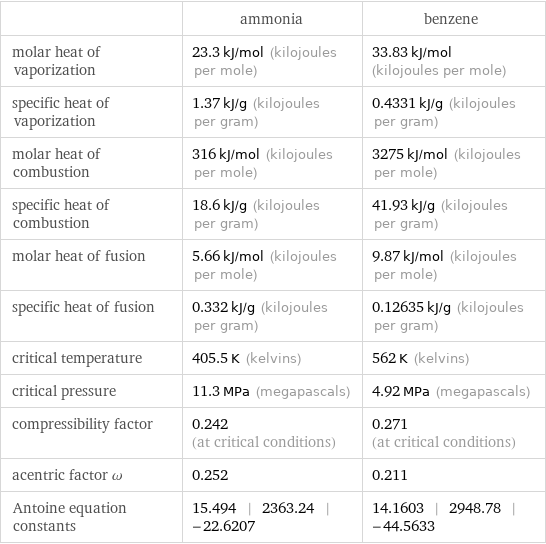
| ammonia | benzene molar heat of vaporization | 23.3 kJ/mol (kilojoules per mole) | 33.83 kJ/mol (kilojoules per mole) specific heat of vaporization | 1.37 kJ/g (kilojoules per gram) | 0.4331 kJ/g (kilojoules per gram) molar heat of combustion | 316 kJ/mol (kilojoules per mole) | 3275 kJ/mol (kilojoules per mole) specific heat of combustion | 18.6 kJ/g (kilojoules per gram) | 41.93 kJ/g (kilojoules per gram) molar heat of fusion | 5.66 kJ/mol (kilojoules per mole) | 9.87 kJ/mol (kilojoules per mole) specific heat of fusion | 0.332 kJ/g (kilojoules per gram) | 0.12635 kJ/g (kilojoules per gram) critical temperature | 405.5 K (kelvins) | 562 K (kelvins) critical pressure | 11.3 MPa (megapascals) | 4.92 MPa (megapascals) compressibility factor | 0.242 (at critical conditions) | 0.271 (at critical conditions) acentric factor ω | 0.252 | 0.211 Antoine equation constants | 15.494 | 2363.24 | -22.6207 | 14.1603 | 2948.78 | -44.5633