Input interpretation

polyatomic ions | formal charges
Table
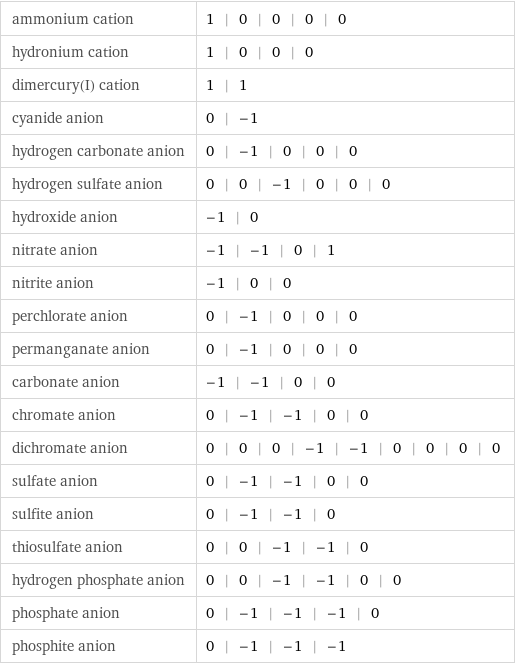
ammonium cation | 1 | 0 | 0 | 0 | 0 hydronium cation | 1 | 0 | 0 | 0 dimercury(I) cation | 1 | 1 cyanide anion | 0 | -1 hydrogen carbonate anion | 0 | -1 | 0 | 0 | 0 hydrogen sulfate anion | 0 | 0 | -1 | 0 | 0 | 0 hydroxide anion | -1 | 0 nitrate anion | -1 | -1 | 0 | 1 nitrite anion | -1 | 0 | 0 perchlorate anion | 0 | -1 | 0 | 0 | 0 permanganate anion | 0 | -1 | 0 | 0 | 0 carbonate anion | -1 | -1 | 0 | 0 chromate anion | 0 | -1 | -1 | 0 | 0 dichromate anion | 0 | 0 | 0 | -1 | -1 | 0 | 0 | 0 | 0 sulfate anion | 0 | -1 | -1 | 0 | 0 sulfite anion | 0 | -1 | -1 | 0 thiosulfate anion | 0 | 0 | -1 | -1 | 0 hydrogen phosphate anion | 0 | 0 | -1 | -1 | 0 | 0 phosphate anion | 0 | -1 | -1 | -1 | 0 phosphite anion | 0 | -1 | -1 | -1