Input interpretation
arsenic(III) sulfide | vanadium tetrachloride
Chemical names and formulas
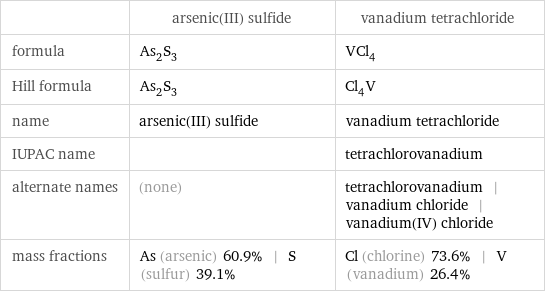
| arsenic(III) sulfide | vanadium tetrachloride formula | As_2S_3 | VCl_4 Hill formula | As_2S_3 | Cl_4V name | arsenic(III) sulfide | vanadium tetrachloride IUPAC name | | tetrachlorovanadium alternate names | (none) | tetrachlorovanadium | vanadium chloride | vanadium(IV) chloride mass fractions | As (arsenic) 60.9% | S (sulfur) 39.1% | Cl (chlorine) 73.6% | V (vanadium) 26.4%
Structure diagrams
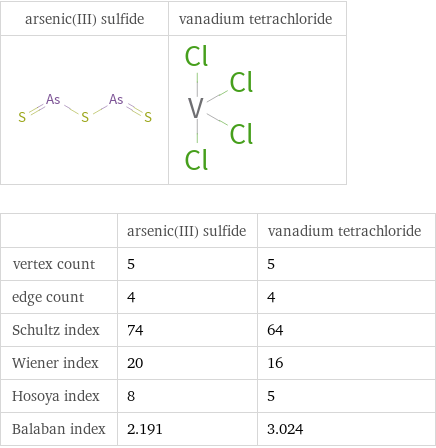
| arsenic(III) sulfide | vanadium tetrachloride vertex count | 5 | 5 edge count | 4 | 4 Schultz index | 74 | 64 Wiener index | 20 | 16 Hosoya index | 8 | 5 Balaban index | 2.191 | 3.024
Basic properties
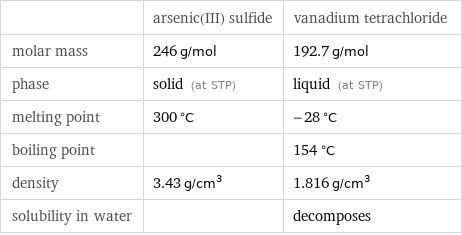
| arsenic(III) sulfide | vanadium tetrachloride molar mass | 246 g/mol | 192.7 g/mol phase | solid (at STP) | liquid (at STP) melting point | 300 °C | -28 °C boiling point | | 154 °C density | 3.43 g/cm^3 | 1.816 g/cm^3 solubility in water | | decomposes
Units

Liquid properties

| vanadium tetrachloride density | 1.816 g/cm^3 vapor pressure | 8 mmHg
Units

Thermodynamic properties

| arsenic(III) sulfide | vanadium tetrachloride specific heat of fusion | 0.117 kJ/g (kilojoules per gram) | 0.012 kJ/g (kilojoules per gram)
Solid properties

| arsenic(III) sulfide density | 3.43 g/cm^3
Units

Chemical identifiers
![| arsenic(III) sulfide | vanadium tetrachloride CAS number | 1303-33-9 | 7632-51-1 PubChem CID number | 4093503 | 24273 PubChem SID number | 24852111 | 24862677 SMILES identifier | S=[As]S[As]=S | Cl[V](Cl)(Cl)Cl](../image_source/3a76cd531dec650b234fbf1b6b30bd7d.png)
| arsenic(III) sulfide | vanadium tetrachloride CAS number | 1303-33-9 | 7632-51-1 PubChem CID number | 4093503 | 24273 PubChem SID number | 24852111 | 24862677 SMILES identifier | S=[As]S[As]=S | Cl[V](Cl)(Cl)Cl