Input interpretation
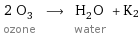
2 O_3 ozone ⟶ H_2O water + K2
Chemical names and formulas
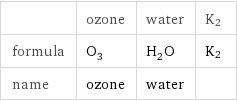
| ozone | water | K2 formula | O_3 | H_2O | K2 name | ozone | water |
Substance properties
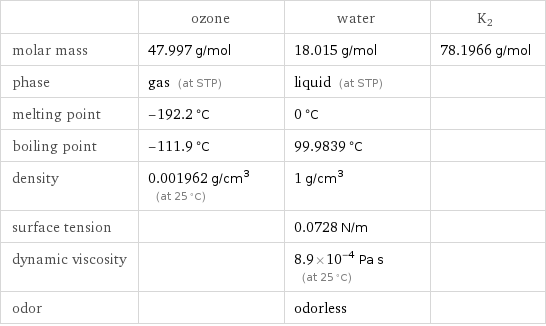
| ozone | water | K2 molar mass | 47.997 g/mol | 18.015 g/mol | 78.1966 g/mol phase | gas (at STP) | liquid (at STP) | melting point | -192.2 °C | 0 °C | boiling point | -111.9 °C | 99.9839 °C | density | 0.001962 g/cm^3 (at 25 °C) | 1 g/cm^3 | surface tension | | 0.0728 N/m | dynamic viscosity | | 8.9×10^-4 Pa s (at 25 °C) | odor | | odorless |
Units
