Input interpretation
strontium hydride | sodium bromate
Chemical names and formulas
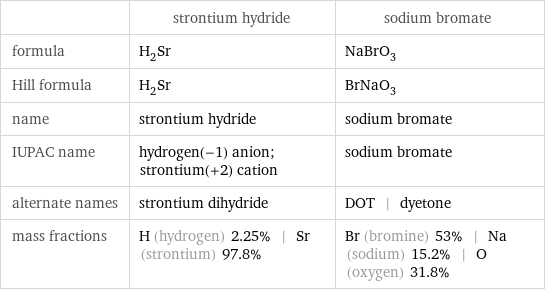
| strontium hydride | sodium bromate formula | H_2Sr | NaBrO_3 Hill formula | H_2Sr | BrNaO_3 name | strontium hydride | sodium bromate IUPAC name | hydrogen(-1) anion; strontium(+2) cation | sodium bromate alternate names | strontium dihydride | DOT | dyetone mass fractions | H (hydrogen) 2.25% | Sr (strontium) 97.8% | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8%
Structure diagrams
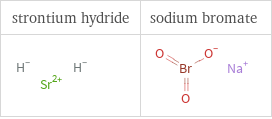
Structure diagrams
Basic properties
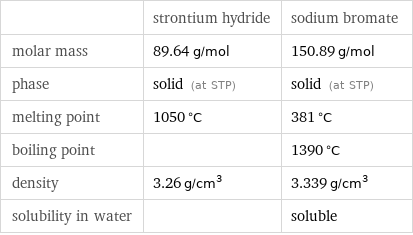
| strontium hydride | sodium bromate molar mass | 89.64 g/mol | 150.89 g/mol phase | solid (at STP) | solid (at STP) melting point | 1050 °C | 381 °C boiling point | | 1390 °C density | 3.26 g/cm^3 | 3.339 g/cm^3 solubility in water | | soluble
Units

Thermodynamic properties
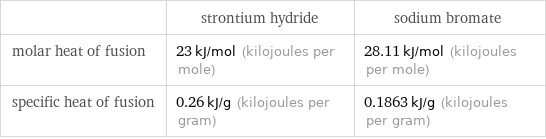
| strontium hydride | sodium bromate molar heat of fusion | 23 kJ/mol (kilojoules per mole) | 28.11 kJ/mol (kilojoules per mole) specific heat of fusion | 0.26 kJ/g (kilojoules per gram) | 0.1863 kJ/g (kilojoules per gram)