Input interpretation
sodium molybdate
Chemical names and formulas

formula | Na_2MoO_4 Hill formula | MoNa_2O_4 name | sodium molybdate IUPAC name | disodium dioxido-dioxomolybdenum alternate names | disodium diketo-dioxido-molybdenum | disodium dioxido-dioxo-molybdenum | disodium dioxido-dioxomolybdenum | disodium molybdate | molybdenum sodium oxide | sodium molybdate(VI) mass fractions | Mo (molybdenum) 46.6% | Na (sodium) 22.3% | O (oxygen) 31.1%
Structure diagram
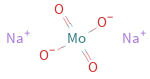
Structure diagram
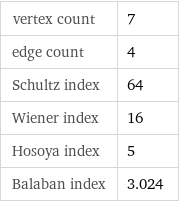
vertex count | 7 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
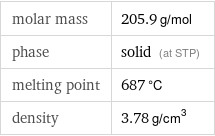
molar mass | 205.9 g/mol phase | solid (at STP) melting point | 687 °C density | 3.78 g/cm^3
Units
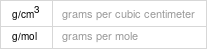
Solid properties (at STP)

density | 3.78 g/cm^3 refractive index | 1.714
Units

Thermodynamic properties
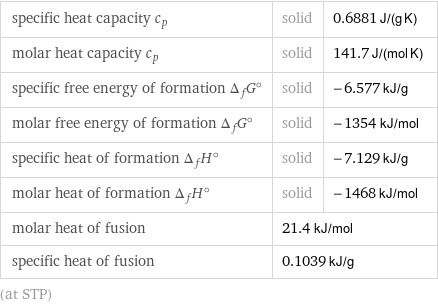
specific heat capacity c_p | solid | 0.6881 J/(g K) molar heat capacity c_p | solid | 141.7 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.577 kJ/g molar free energy of formation Δ_fG° | solid | -1354 kJ/mol specific heat of formation Δ_fH° | solid | -7.129 kJ/g molar heat of formation Δ_fH° | solid | -1468 kJ/mol molar heat of fusion | 21.4 kJ/mol | specific heat of fusion | 0.1039 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7631-95-0 PubChem CID number | 4384450 PubChem SID number | 24854628 SMILES identifier | [O-][Mo](=O)(=O)[O-].[Na+].[Na+] InChI identifier | InChI=1/Mo.2Na.4O/q;2*+1;;;2*-1/rMoO4.2Na/c2-1(3, 4)5;;/q-2;2*+1 RTECS number | QA5075000 MDL number | MFCD00003486](../image_source/5fa6d6fd7d33446925927ef673c09111.png)
CAS number | 7631-95-0 PubChem CID number | 4384450 PubChem SID number | 24854628 SMILES identifier | [O-][Mo](=O)(=O)[O-].[Na+].[Na+] InChI identifier | InChI=1/Mo.2Na.4O/q;2*+1;;;2*-1/rMoO4.2Na/c2-1(3, 4)5;;/q-2;2*+1 RTECS number | QA5075000 MDL number | MFCD00003486
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties
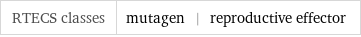
RTECS classes | mutagen | reproductive effector
Ion equivalents

Na^+ (sodium cation) | 2 (MoO_4)^(2-) (molybdate anion) | 1