Input interpretation
ferrous sulfide
Chemical names and formulas

formula | FeS name | ferrous sulfide alternate names | ferrous sulfide (iron(II) sulfide) | iron(II) sulfide | iron monosulfide | sulfanylidene iron | thioxoiron mass fractions | Fe (iron) 63.5% | S (sulfur) 36.5%
Structure diagram
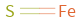
Structure diagram
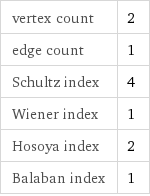
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
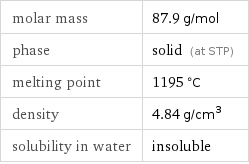
molar mass | 87.9 g/mol phase | solid (at STP) melting point | 1195 °C density | 4.84 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 4.84 g/cm^3 vapor pressure | 1.1145 mmHg (at 1300 °C)
Units

Thermodynamic properties
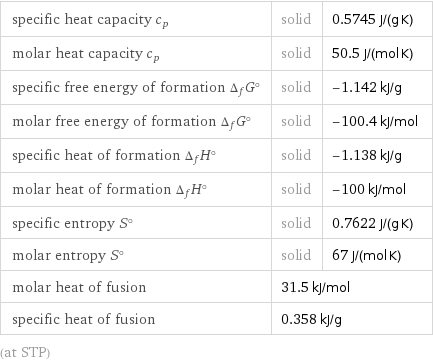
specific heat capacity c_p | solid | 0.5745 J/(g K) molar heat capacity c_p | solid | 50.5 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.142 kJ/g molar free energy of formation Δ_fG° | solid | -100.4 kJ/mol specific heat of formation Δ_fH° | solid | -1.138 kJ/g molar heat of formation Δ_fH° | solid | -100 kJ/mol specific entropy S° | solid | 0.7622 J/(g K) molar entropy S° | solid | 67 J/(mol K) molar heat of fusion | 31.5 kJ/mol | specific heat of fusion | 0.358 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1317-37-9 PubChem CID number | 14828 SMILES identifier | [Fe]=S MDL number | MFCD00011013](../image_source/dbeb49e3a84bf53e9f5ab201c9de8c4e.png)
CAS number | 1317-37-9 PubChem CID number | 14828 SMILES identifier | [Fe]=S MDL number | MFCD00011013