Input interpretation
Peng-Robinson equation of state
Input values
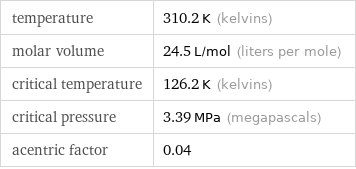
temperature | 310.2 K (kelvins) molar volume | 24.5 L/mol (liters per mole) critical temperature | 126.2 K (kelvins) critical pressure | 3.39 MPa (megapascals) acentric factor | 0.04
Result
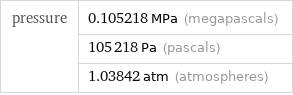
pressure | 0.105218 MPa (megapascals) | 105218 Pa (pascals) | 1.03842 atm (atmospheres)
Formula
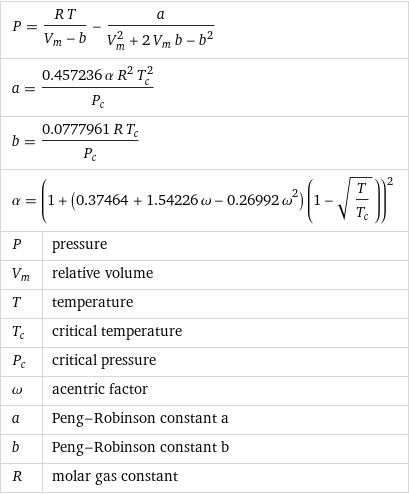
P = (R T)/(V_m - b) - a/(V_m^2 + 2 V_m b - b^2) | a = (0.457236 α R^2 T_c^2)/P_c | b = (0.0777961 R T_c)/P_c | α = (1 + (0.37464 + 1.54226 ω - 0.26992 ω^2) (1 - sqrt(T/T_c)))^2 | P | pressure V_m | relative volume T | temperature T_c | critical temperature P_c | critical pressure ω | acentric factor a | Peng-Robinson constant a b | Peng-Robinson constant b R | molar gas constant
Phase diagram
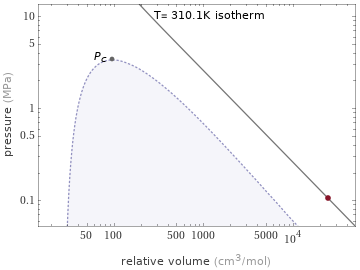
Phase diagram