Input interpretation
silicon monoxide
Chemical names and formulas
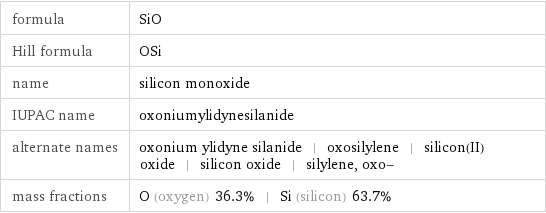
formula | SiO Hill formula | OSi name | silicon monoxide IUPAC name | oxoniumylidynesilanide alternate names | oxonium ylidyne silanide | oxosilylene | silicon(II) oxide | silicon oxide | silylene, oxo- mass fractions | O (oxygen) 36.3% | Si (silicon) 63.7%
Lewis structure

Draw the Lewis structure of silicon monoxide. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the oxygen (n_O, val = 6) and silicon (n_Si, val = 4) atoms: n_O, val + n_Si, val = 10 Calculate the number of electrons needed to completely fill the valence shells for oxygen (n_O, full = 8) and silicon (n_Si, full = 8): n_O, full + n_Si, full = 16 Subtracting these two numbers shows that 16 - 10 = 6 bonding electrons are needed. Each bond has two electrons, so in addition to the 1 bond already present in the diagram add 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 2 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. The triple bond is a dipolar bond with oxygen donating both electrons: Answer: | |
3D structure

3D structure
Basic properties
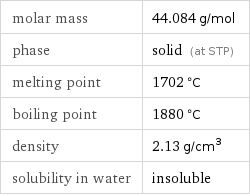
molar mass | 44.084 g/mol phase | solid (at STP) melting point | 1702 °C boiling point | 1880 °C density | 2.13 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 2.13 g/cm^3
Units

Thermodynamic properties

specific free energy of formation Δ_fG° | gas | -2.867 kJ/g specific heat of formation Δ_fH° | gas | -2.259 kJ/g (at STP)
Units

Chemical identifiers
![CAS number | 10097-28-6 PubChem CID number | 66241 SMILES identifier | [O+]#[Si-]](../image_source/cb013f87ff7648096b4db65dcca1c06c.png)
CAS number | 10097-28-6 PubChem CID number | 66241 SMILES identifier | [O+]#[Si-]
NFPA label
NFPA label