Input interpretation
1, 1'-dioctadecyl-4, 4'-bipyridinium diperchlorate
Basic properties
![molar mass | 862.1 g/mol formula | C_46H_82Cl_2N_2O_8 empirical formula | Cl_O_4C_23N_H_41 SMILES identifier | CCCCCCCCCCCCCCCCCC[N+]1=CC=C(C=C1)C2=CC=[N+](CCCCCCCCCCCCCCCCCC)C=C2.Cl(=O)(=O)(=O)[O-].Cl(=O)(=O)(=O)[O-] InChI identifier | InChI=1/C46H82N2.2ClHO4/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-39-47-41-35-45(36-42-47)46-37-43-48(44-38-46)40-34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2;2*2-1(3, 4)5/h35-38, 41-44H, 3-34, 39-40H2, 1-2H3;2*(H, 2, 3, 4, 5)/q+2;;/p-2/fC46H82N2.2ClO4/qm;2*-1 InChI key | MDOXEKFXLCHCDC-UHFFFAOYSA-L](../image_source/b978585ec7781c444a3c675dc2856007.png)
molar mass | 862.1 g/mol formula | C_46H_82Cl_2N_2O_8 empirical formula | Cl_O_4C_23N_H_41 SMILES identifier | CCCCCCCCCCCCCCCCCC[N+]1=CC=C(C=C1)C2=CC=[N+](CCCCCCCCCCCCCCCCCC)C=C2.Cl(=O)(=O)(=O)[O-].Cl(=O)(=O)(=O)[O-] InChI identifier | InChI=1/C46H82N2.2ClHO4/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-39-47-41-35-45(36-42-47)46-37-43-48(44-38-46)40-34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2;2*2-1(3, 4)5/h35-38, 41-44H, 3-34, 39-40H2, 1-2H3;2*(H, 2, 3, 4, 5)/q+2;;/p-2/fC46H82N2.2ClO4/qm;2*-1 InChI key | MDOXEKFXLCHCDC-UHFFFAOYSA-L
Structure diagram
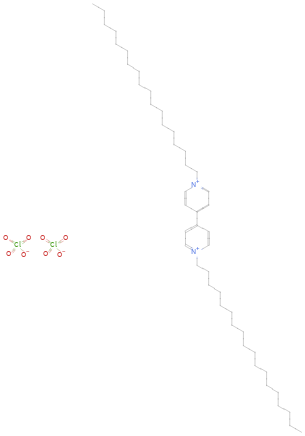
Structure diagram
Quantitative molecular descriptors
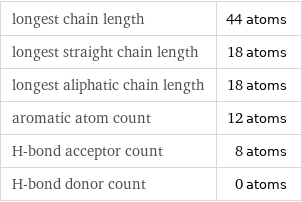
longest chain length | 44 atoms longest straight chain length | 18 atoms longest aliphatic chain length | 18 atoms aromatic atom count | 12 atoms H-bond acceptor count | 8 atoms H-bond donor count | 0 atoms
Elemental composition
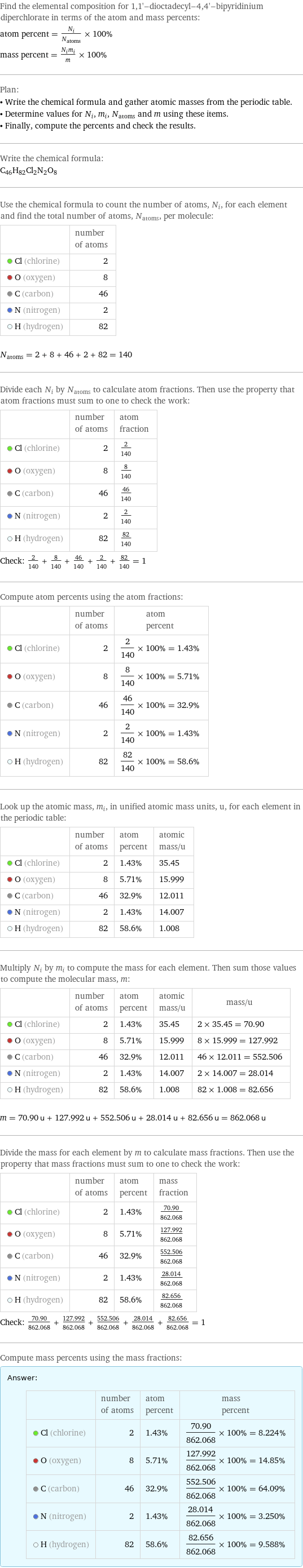
Find the elemental composition for 1, 1'-dioctadecyl-4, 4'-bipyridinium diperchlorate in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_46H_82Cl_2N_2O_8 Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms Cl (chlorine) | 2 O (oxygen) | 8 C (carbon) | 46 N (nitrogen) | 2 H (hydrogen) | 82 N_atoms = 2 + 8 + 46 + 2 + 82 = 140 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction Cl (chlorine) | 2 | 2/140 O (oxygen) | 8 | 8/140 C (carbon) | 46 | 46/140 N (nitrogen) | 2 | 2/140 H (hydrogen) | 82 | 82/140 Check: 2/140 + 8/140 + 46/140 + 2/140 + 82/140 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent Cl (chlorine) | 2 | 2/140 × 100% = 1.43% O (oxygen) | 8 | 8/140 × 100% = 5.71% C (carbon) | 46 | 46/140 × 100% = 32.9% N (nitrogen) | 2 | 2/140 × 100% = 1.43% H (hydrogen) | 82 | 82/140 × 100% = 58.6% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u Cl (chlorine) | 2 | 1.43% | 35.45 O (oxygen) | 8 | 5.71% | 15.999 C (carbon) | 46 | 32.9% | 12.011 N (nitrogen) | 2 | 1.43% | 14.007 H (hydrogen) | 82 | 58.6% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u Cl (chlorine) | 2 | 1.43% | 35.45 | 2 × 35.45 = 70.90 O (oxygen) | 8 | 5.71% | 15.999 | 8 × 15.999 = 127.992 C (carbon) | 46 | 32.9% | 12.011 | 46 × 12.011 = 552.506 N (nitrogen) | 2 | 1.43% | 14.007 | 2 × 14.007 = 28.014 H (hydrogen) | 82 | 58.6% | 1.008 | 82 × 1.008 = 82.656 m = 70.90 u + 127.992 u + 552.506 u + 28.014 u + 82.656 u = 862.068 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction Cl (chlorine) | 2 | 1.43% | 70.90/862.068 O (oxygen) | 8 | 5.71% | 127.992/862.068 C (carbon) | 46 | 32.9% | 552.506/862.068 N (nitrogen) | 2 | 1.43% | 28.014/862.068 H (hydrogen) | 82 | 58.6% | 82.656/862.068 Check: 70.90/862.068 + 127.992/862.068 + 552.506/862.068 + 28.014/862.068 + 82.656/862.068 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent Cl (chlorine) | 2 | 1.43% | 70.90/862.068 × 100% = 8.224% O (oxygen) | 8 | 5.71% | 127.992/862.068 × 100% = 14.85% C (carbon) | 46 | 32.9% | 552.506/862.068 × 100% = 64.09% N (nitrogen) | 2 | 1.43% | 28.014/862.068 × 100% = 3.250% H (hydrogen) | 82 | 58.6% | 82.656/862.068 × 100% = 9.588%