Input interpretation

period 4 elements | atomic radius
Summary
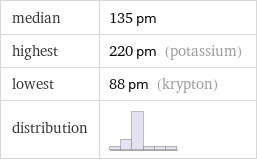
median | 135 pm highest | 220 pm (potassium) lowest | 88 pm (krypton) distribution |
Units

Plot
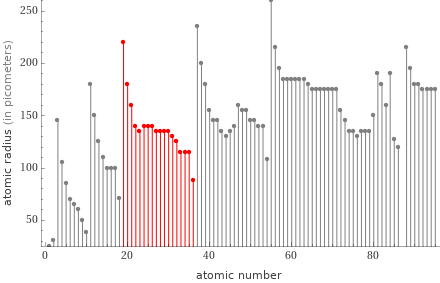
Plot
Periodic table location
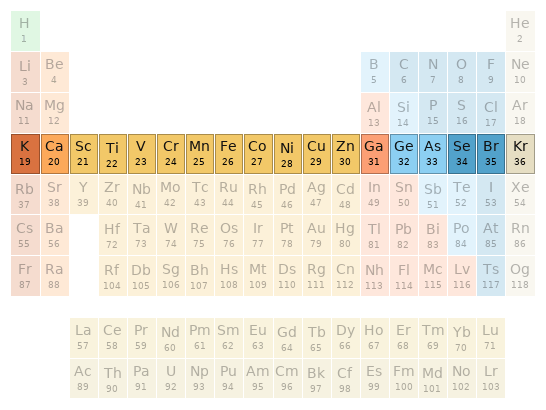
Periodic table location
Atomic radii properties
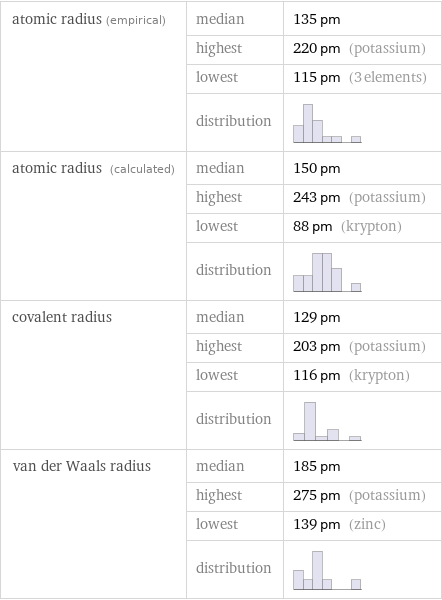
atomic radius (empirical) | median | 135 pm | highest | 220 pm (potassium) | lowest | 115 pm (3 elements) | distribution | atomic radius (calculated) | median | 150 pm | highest | 243 pm (potassium) | lowest | 88 pm (krypton) | distribution | covalent radius | median | 129 pm | highest | 203 pm (potassium) | lowest | 116 pm (krypton) | distribution | van der Waals radius | median | 185 pm | highest | 275 pm (potassium) | lowest | 139 pm (zinc) | distribution |
Units

Distribution plots
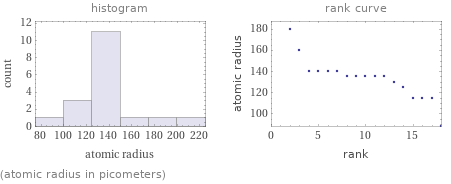
(atomic radius in picometers)
Atomic radius rankings
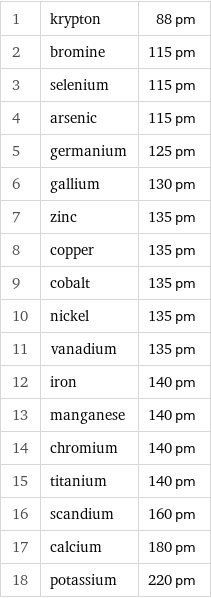
1 | krypton | 88 pm 2 | bromine | 115 pm 3 | selenium | 115 pm 4 | arsenic | 115 pm 5 | germanium | 125 pm 6 | gallium | 130 pm 7 | zinc | 135 pm 8 | copper | 135 pm 9 | cobalt | 135 pm 10 | nickel | 135 pm 11 | vanadium | 135 pm 12 | iron | 140 pm 13 | manganese | 140 pm 14 | chromium | 140 pm 15 | titanium | 140 pm 16 | scandium | 160 pm 17 | calcium | 180 pm 18 | potassium | 220 pm
Unit conversions for median atomic radius 135 pm
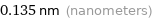
0.135 nm (nanometers)
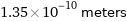
1.35×10^-10 meters
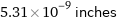
5.31×10^-9 inches
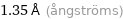
1.35 Å (ångströms)
Comparisons for median atomic radius 135 pm
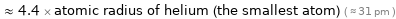
≈ 4.4 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
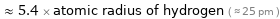
≈ 5.4 × atomic radius of hydrogen ( ≈ 25 pm )