Input interpretation
lime | sodium fluorosilicate
Chemical names and formulas
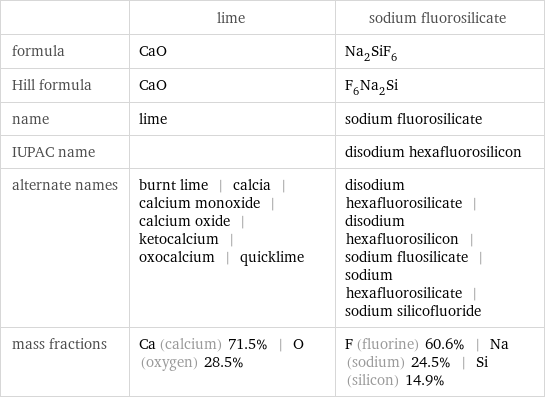
| lime | sodium fluorosilicate formula | CaO | Na_2SiF_6 Hill formula | CaO | F_6Na_2Si name | lime | sodium fluorosilicate IUPAC name | | disodium hexafluorosilicon alternate names | burnt lime | calcia | calcium monoxide | calcium oxide | ketocalcium | oxocalcium | quicklime | disodium hexafluorosilicate | disodium hexafluorosilicon | sodium fluosilicate | sodium hexafluorosilicate | sodium silicofluoride mass fractions | Ca (calcium) 71.5% | O (oxygen) 28.5% | F (fluorine) 60.6% | Na (sodium) 24.5% | Si (silicon) 14.9%
Structure diagrams
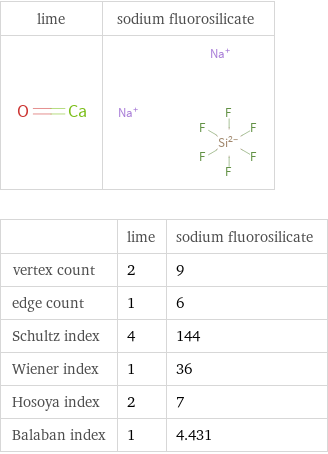
| lime | sodium fluorosilicate vertex count | 2 | 9 edge count | 1 | 6 Schultz index | 4 | 144 Wiener index | 1 | 36 Hosoya index | 2 | 7 Balaban index | 1 | 4.431
Basic properties
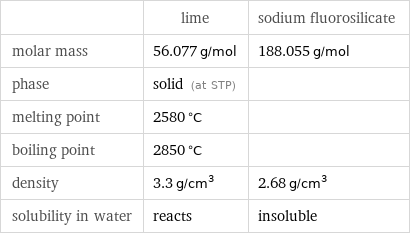
| lime | sodium fluorosilicate molar mass | 56.077 g/mol | 188.055 g/mol phase | solid (at STP) | melting point | 2580 °C | boiling point | 2850 °C | density | 3.3 g/cm^3 | 2.68 g/cm^3 solubility in water | reacts | insoluble
Units

Thermodynamic properties
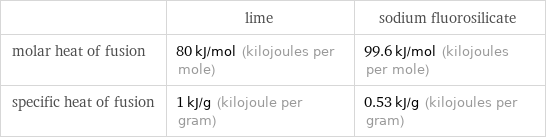
| lime | sodium fluorosilicate molar heat of fusion | 80 kJ/mol (kilojoules per mole) | 99.6 kJ/mol (kilojoules per mole) specific heat of fusion | 1 kJ/g (kilojoule per gram) | 0.53 kJ/g (kilojoules per gram)