Input interpretation

period 5 elements | molar heat of vaporization
Summary
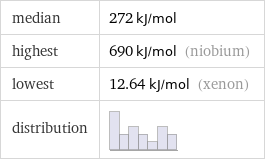
median | 272 kJ/mol highest | 690 kJ/mol (niobium) lowest | 12.64 kJ/mol (xenon) distribution |
Units

Distribution plots
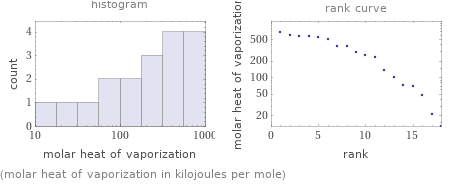
(molar heat of vaporization in kilojoules per mole)
Molar heat of vaporization rankings
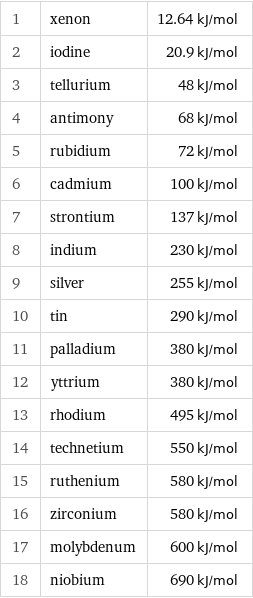
1 | xenon | 12.64 kJ/mol 2 | iodine | 20.9 kJ/mol 3 | tellurium | 48 kJ/mol 4 | antimony | 68 kJ/mol 5 | rubidium | 72 kJ/mol 6 | cadmium | 100 kJ/mol 7 | strontium | 137 kJ/mol 8 | indium | 230 kJ/mol 9 | silver | 255 kJ/mol 10 | tin | 290 kJ/mol 11 | palladium | 380 kJ/mol 12 | yttrium | 380 kJ/mol 13 | rhodium | 495 kJ/mol 14 | technetium | 550 kJ/mol 15 | ruthenium | 580 kJ/mol 16 | zirconium | 580 kJ/mol 17 | molybdenum | 600 kJ/mol 18 | niobium | 690 kJ/mol
Unit conversions for median molar heat of vaporization 272 kJ/mol
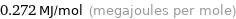
0.272 MJ/mol (megajoules per mole)
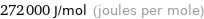
272000 J/mol (joules per mole)

65.1 kcal/mol (thermochemical kilocalories per mole)
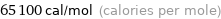
65100 cal/mol (calories per mole)
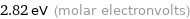
2.82 eV (molar electronvolts)