Input interpretation

carbon | phase at STP
Result
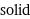
solid
Thermodynamic properties
![phase at STP | solid | melting point | [5, 6]-fullerene-C70 | 280 °C | buckminsterfullerene | 280 °C | activated charcoal | 3550 °C | graphite | 3675 °C boiling point | activated charcoal | 4027 °C molar heat of fusion | graphite | 117.4 kJ/mol molar heat of vaporization | 715 kJ/mol (rank: 3rd) | specific heat at STP | 710 J/(kg K) (rank: 16th) | (properties at standard conditions)](../image_source/e4927cfcdbb3c698b2b040cac67c1ae0.png)
phase at STP | solid | melting point | [5, 6]-fullerene-C70 | 280 °C | buckminsterfullerene | 280 °C | activated charcoal | 3550 °C | graphite | 3675 °C boiling point | activated charcoal | 4027 °C molar heat of fusion | graphite | 117.4 kJ/mol molar heat of vaporization | 715 kJ/mol (rank: 3rd) | specific heat at STP | 710 J/(kg K) (rank: 16th) | (properties at standard conditions)