Input interpretation

period 1 elements | atomic mass
Results

hydrogen | 1.008 u (unified atomic mass units) helium | 4.002602 u (unified atomic mass units)
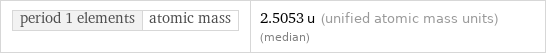
period 1 elements | atomic mass | 2.5053 u (unified atomic mass units) (median)
Relative values
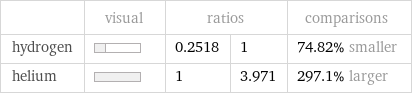
| visual | ratios | | comparisons hydrogen | | 0.2518 | 1 | 74.82% smaller helium | | 1 | 3.971 | 297.1% larger
Unit conversions for median atomic mass 2.5053 u
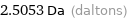
2.5053 Da (daltons)
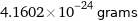
4.1602×10^-24 grams
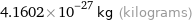
4.1602×10^-27 kg (kilograms)
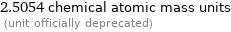
2.5054 chemical atomic mass units (unit officially deprecated)
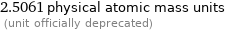
2.5061 physical atomic mass units (unit officially deprecated)
Corresponding quantities
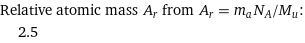
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 2.5
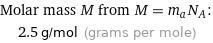
Molar mass M from M = m_aN_A: | 2.5 g/mol (grams per mole)