Input interpretation

non-polar solvents | molar heat of fusion
Summary
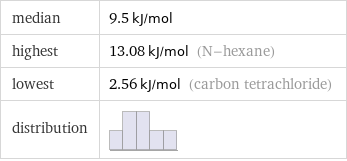
median | 9.5 kJ/mol highest | 13.08 kJ/mol (N-hexane) lowest | 2.56 kJ/mol (carbon tetrachloride) distribution |
Units

Ranked values
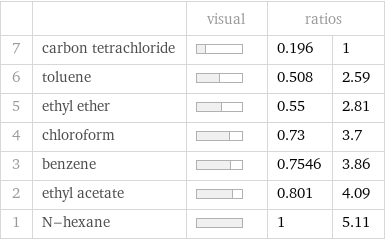
| | visual | ratios | 7 | carbon tetrachloride | | 0.196 | 1 6 | toluene | | 0.508 | 2.59 5 | ethyl ether | | 0.55 | 2.81 4 | chloroform | | 0.73 | 3.7 3 | benzene | | 0.7546 | 3.86 2 | ethyl acetate | | 0.801 | 4.09 1 | N-hexane | | 1 | 5.11
Molar heat of fusion rankings
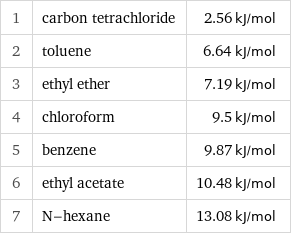
1 | carbon tetrachloride | 2.56 kJ/mol 2 | toluene | 6.64 kJ/mol 3 | ethyl ether | 7.19 kJ/mol 4 | chloroform | 9.5 kJ/mol 5 | benzene | 9.87 kJ/mol 6 | ethyl acetate | 10.48 kJ/mol 7 | N-hexane | 13.08 kJ/mol
Unit conversions for median molar heat of fusion 9.5 kJ/mol
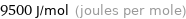
9500 J/mol (joules per mole)
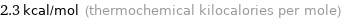
2.3 kcal/mol (thermochemical kilocalories per mole)
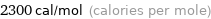
2300 cal/mol (calories per mole)

0.098 eV (molar electronvolts)