Input interpretation
2 kg of N-hexane
Basic properties for 2 kg
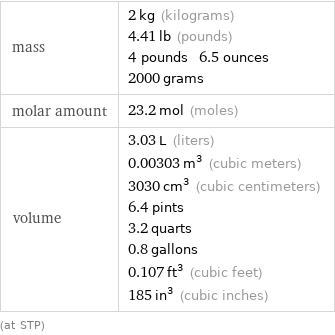
mass | 2 kg (kilograms) 4.41 lb (pounds) 4 pounds 6.5 ounces 2000 grams molar amount | 23.2 mol (moles) volume | 3.03 L (liters) 0.00303 m^3 (cubic meters) 3030 cm^3 (cubic centimeters) 6.4 pints 3.2 quarts 0.8 gallons 0.107 ft^3 (cubic feet) 185 in^3 (cubic inches) (at STP)
Corresponding quantities
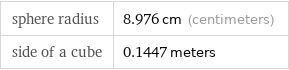
sphere radius | 8.976 cm (centimeters) side of a cube | 0.1447 meters
Thermodynamic properties for 2 kg

enthalpy of hydration | -740.3 kJ (kilojoules) | energy content | 96.6 MJ (megajoules) | heat capacity C_p | liquid | 4539 J/K heat of formation Δ_fH° | gaseous | -3873 kJ latent heat of vaporization | 757 kJ (kilojoules) | latent heat of fusion | 304 kJ (kilojoules) |
Units
Energy vs. temperature for 2 kg
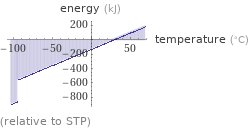
(relative to STP)
Units

Phase change energies for 2 kg from 25 °C

energy required to heat to boiling point | 199 kJ (kilojoules) energy required to convert to vapor | 757 kJ (kilojoules) energy required to heat to boiling point and convert to vapor | 955 kJ (kilojoules) energy released from cooling to freezing point | 546 kJ (kilojoules) energy released from converting to solid | 304 kJ (kilojoules) energy released from cooling to freezing point and converting to solid | 850 kJ (kilojoules)
Mass composition for 2 kg
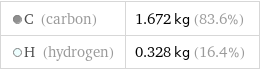
C (carbon) | 1.672 kg (83.6%) H (hydrogen) | 0.328 kg (16.4%)
Mass composition for 2 kg
Lewis structure
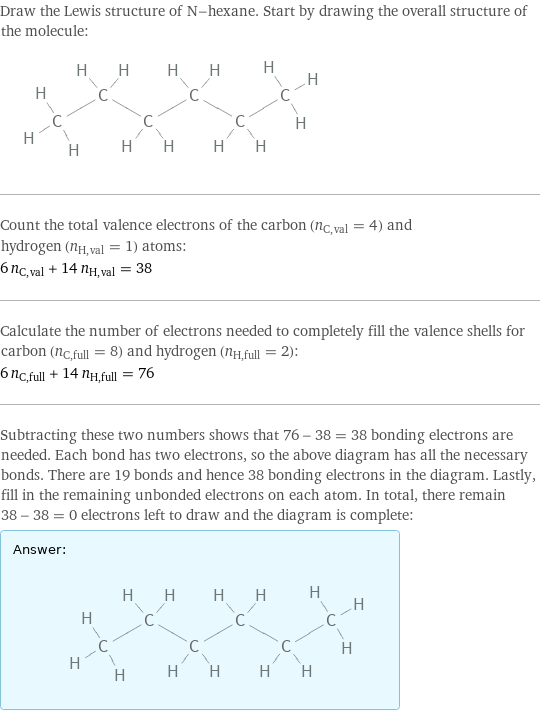
Draw the Lewis structure of N-hexane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4) and hydrogen (n_H, val = 1) atoms: 6 n_C, val + 14 n_H, val = 38 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8) and hydrogen (n_H, full = 2): 6 n_C, full + 14 n_H, full = 76 Subtracting these two numbers shows that 76 - 38 = 38 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 19 bonds and hence 38 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 38 - 38 = 0 electrons left to draw and the diagram is complete: Answer: | |
Chemical names and formulas
formula | CH_3(CH_2)_4CH_3 Hill formula | C_6H_14 name | N-hexane IUPAC name | hexane
Substance properties
molar mass | 86.18 g/mol phase | liquid (at STP) melting point | -95.35 °C boiling point | 68.73 °C density | 0.6603 g/cm^3 surface tension | 0.0184 N/m dynamic viscosity | 3×10^-4 Pa s (at 25 °C) odor | gasoline-like odor threshold | 130 ppm
Units
