Input interpretation

oxidants | molecular mass
Summary
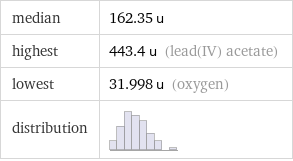
median | 162.35 u highest | 443.4 u (lead(IV) acetate) lowest | 31.998 u (oxygen) distribution |
Units

Distribution plots
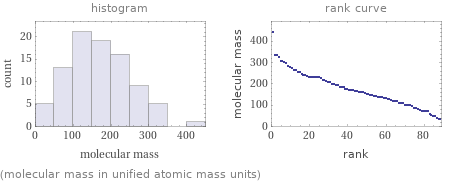
(molecular mass in unified atomic mass units)
Molecular mass rankings
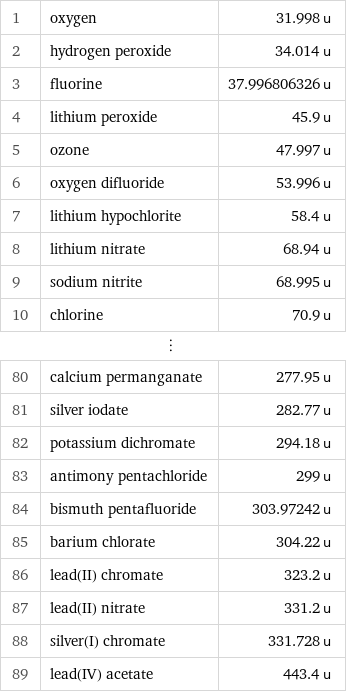
1 | oxygen | 31.998 u 2 | hydrogen peroxide | 34.014 u 3 | fluorine | 37.996806326 u 4 | lithium peroxide | 45.9 u 5 | ozone | 47.997 u 6 | oxygen difluoride | 53.996 u 7 | lithium hypochlorite | 58.4 u 8 | lithium nitrate | 68.94 u 9 | sodium nitrite | 68.995 u 10 | chlorine | 70.9 u ⋮ | | 80 | calcium permanganate | 277.95 u 81 | silver iodate | 282.77 u 82 | potassium dichromate | 294.18 u 83 | antimony pentachloride | 299 u 84 | bismuth pentafluoride | 303.97242 u 85 | barium chlorate | 304.22 u 86 | lead(II) chromate | 323.2 u 87 | lead(II) nitrate | 331.2 u 88 | silver(I) chromate | 331.728 u 89 | lead(IV) acetate | 443.4 u
Unit conversions for median molecular mass 162.35 u
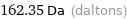
162.35 Da (daltons)
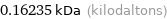
0.16235 kDa (kilodaltons)
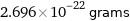
2.696×10^-22 grams
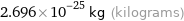
2.696×10^-25 kg (kilograms)

162.36 chemical atomic mass units (unit officially deprecated)
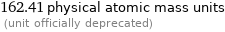
162.41 physical atomic mass units (unit officially deprecated)
Comparisons for median molecular mass 162.35 u

≈ ( 0.23 ≈ 1/4 ) × molecular mass of fullerene ( ≈ 721 u )
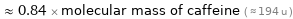
≈ 0.84 × molecular mass of caffeine ( ≈ 194 u )
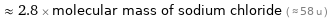
≈ 2.8 × molecular mass of sodium chloride ( ≈ 58 u )
Corresponding quantities
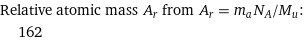
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 162
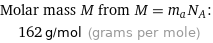
Molar mass M from M = m_aN_A: | 162 g/mol (grams per mole)
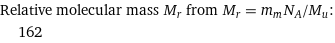
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 162
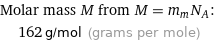
Molar mass M from M = m_mN_A: | 162 g/mol (grams per mole)