Input interpretation

weak bases | molar heat of fusion
Summary

median | 6.6 kJ/mol highest | 12.66 kJ/mol (diazane) lowest | 5.66 kJ/mol (ammonia) distribution | | (based on 8 values; 5 unavailable)
Entities with missing values
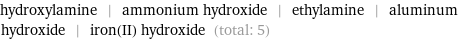
hydroxylamine | ammonium hydroxide | ethylamine | aluminum hydroxide | iron(II) hydroxide (total: 5)
Molar heat of fusion rankings
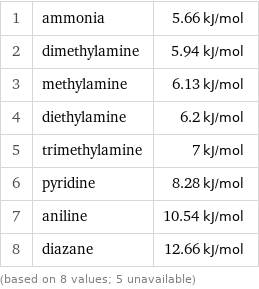
1 | ammonia | 5.66 kJ/mol 2 | dimethylamine | 5.94 kJ/mol 3 | methylamine | 6.13 kJ/mol 4 | diethylamine | 6.2 kJ/mol 5 | trimethylamine | 7 kJ/mol 6 | pyridine | 8.28 kJ/mol 7 | aniline | 10.54 kJ/mol 8 | diazane | 12.66 kJ/mol (based on 8 values; 5 unavailable)
Unit conversions for median molar heat of fusion 6.6 kJ/mol
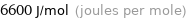
6600 J/mol (joules per mole)
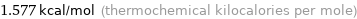
1.577 kcal/mol (thermochemical kilocalories per mole)
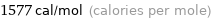
1577 cal/mol (calories per mole)

0.0684 eV (molar electronvolts)