Input interpretation
1 in^3 of barium hydroxide
Basic properties for 1 in^3
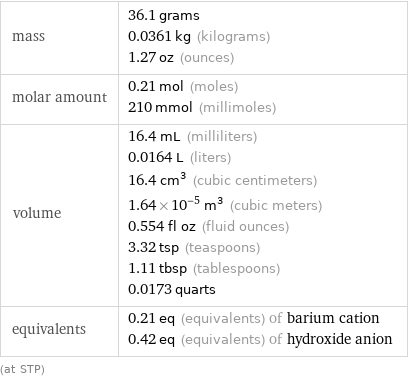
mass | 36.1 grams 0.0361 kg (kilograms) 1.27 oz (ounces) molar amount | 0.21 mol (moles) 210 mmol (millimoles) volume | 16.4 mL (milliliters) 0.0164 L (liters) 16.4 cm^3 (cubic centimeters) 1.64×10^-5 m^3 (cubic meters) 0.554 fl oz (fluid ounces) 3.32 tsp (teaspoons) 1.11 tbsp (tablespoons) 0.0173 quarts equivalents | 0.21 eq (equivalents) of barium cation 0.42 eq (equivalents) of hydroxide anion (at STP)
Corresponding quantities
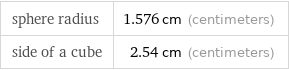
sphere radius | 1.576 cm (centimeters) side of a cube | 2.54 cm (centimeters)
Thermodynamic properties for 1 in^3

heat of formation Δ_fH° | solid | -198.8 kJ latent heat of fusion | 3.37 kJ (kilojoules) |
Units

Phase change energies for 1 in^3 from 25 °C

energy required to convert to liquid | 3.37 kJ (kilojoules)
Mass composition for 1 in^3
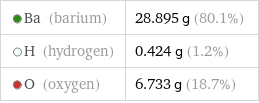
Ba (barium) | 28.895 g (80.1%) H (hydrogen) | 0.424 g (1.2%) O (oxygen) | 6.733 g (18.7%)
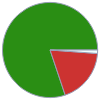
Mass composition for 1 in^3
Structure diagram
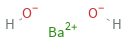
Structure diagram
Chemical names and formulas

formula | Ba(OH)_2 Hill formula | BaH_2O_2 name | barium hydroxide IUPAC name | barium(+2) cation dihydroxide
Substance properties
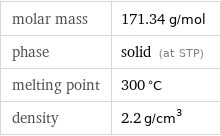
molar mass | 171.34 g/mol phase | solid (at STP) melting point | 300 °C density | 2.2 g/cm^3
Units
