Input interpretation
1 atom of copper (chemical element)
Basic properties for 1 atom
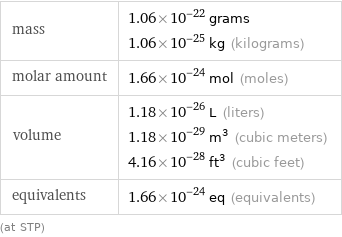
mass | 1.06×10^-22 grams 1.06×10^-25 kg (kilograms) molar amount | 1.66×10^-24 mol (moles) volume | 1.18×10^-26 L (liters) 1.18×10^-29 m^3 (cubic meters) 4.16×10^-28 ft^3 (cubic feet) equivalents | 1.66×10^-24 eq (equivalents) (at STP)
Current commodity price
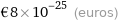
€8×10^-25 (euros)
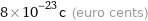
8×10^-23c (euro cents)
Thermodynamic properties for 1 atom
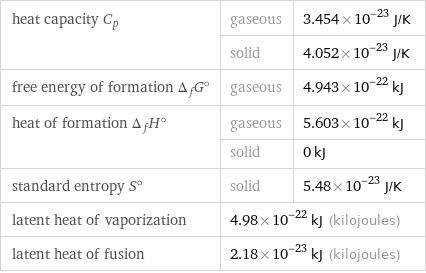
heat capacity C_p | gaseous | 3.454×10^-23 J/K | solid | 4.052×10^-23 J/K free energy of formation Δ_fG° | gaseous | 4.943×10^-22 kJ heat of formation Δ_fH° | gaseous | 5.603×10^-22 kJ | solid | 0 kJ standard entropy S° | solid | 5.48×10^-23 J/K latent heat of vaporization | 4.98×10^-22 kJ (kilojoules) | latent heat of fusion | 2.18×10^-23 kJ (kilojoules) |
Units

Phase change energies for 1 atom from 25 °C

energy required to heat to melting point | 4.29×10^-23 kJ (kilojoules) energy required to convert to liquid | 2.18×10^-23 kJ (kilojoules) energy required to heat to melting point and convert to liquid | 6.47×10^-23 kJ (kilojoules)
Corresponding quantities

sphere radius | 0.1411 nm (nanometers) side of a cube | 0.2275 nm (nanometers)
Structure diagram
Structure diagram
Chemical names and formulas
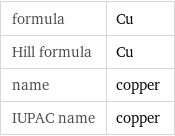
formula | Cu Hill formula | Cu name | copper IUPAC name | copper
Substance properties
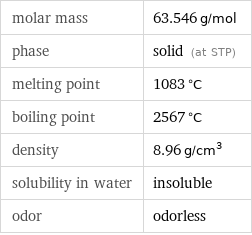
molar mass | 63.546 g/mol phase | solid (at STP) melting point | 1083 °C boiling point | 2567 °C density | 8.96 g/cm^3 solubility in water | insoluble odor | odorless
Units
