Input interpretation
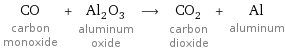
CO carbon monoxide + Al_2O_3 aluminum oxide ⟶ CO_2 carbon dioxide + Al aluminum
Balanced equation

Balance the chemical equation algebraically: CO + Al_2O_3 ⟶ CO_2 + Al Add stoichiometric coefficients, c_i, to the reactants and products: c_1 CO + c_2 Al_2O_3 ⟶ c_3 CO_2 + c_4 Al Set the number of atoms in the reactants equal to the number of atoms in the products for C, O and Al: C: | c_1 = c_3 O: | c_1 + 3 c_2 = 2 c_3 Al: | 2 c_2 = c_4 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_2 = 1 and solve the system of equations for the remaining coefficients: c_1 = 3 c_2 = 1 c_3 = 3 c_4 = 2 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | 3 CO + Al_2O_3 ⟶ 3 CO_2 + 2 Al
Structures
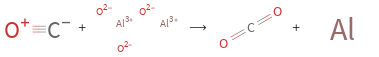
+ ⟶ +
Names
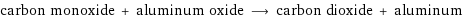
carbon monoxide + aluminum oxide ⟶ carbon dioxide + aluminum
Reaction thermodynamics
Enthalpy

ΔH_rxn^0 | -1181 kJ/mol - -2008 kJ/mol = 827 kJ/mol (endothermic)
Entropy

ΔS_rxn^0 | 698.6 J/(mol K) - 645 J/(mol K) = 53.6 J/(mol K) (endoentropic)
Units

Equilibrium constant
![K_c = ([CO2]^3 [Al]^2)/([CO]^3 [Al2O3])](../image_source/bbbcccb5d35b34b3298cb13aafc75808.png)
K_c = ([CO2]^3 [Al]^2)/([CO]^3 [Al2O3])
Rate of reaction
![rate = -1/3 (Δ[CO])/(Δt) = -(Δ[Al2O3])/(Δt) = 1/3 (Δ[CO2])/(Δt) = 1/2 (Δ[Al])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/f388c15f76ca8ac7caff605b56fd657c.png)
rate = -1/3 (Δ[CO])/(Δt) = -(Δ[Al2O3])/(Δt) = 1/3 (Δ[CO2])/(Δt) = 1/2 (Δ[Al])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
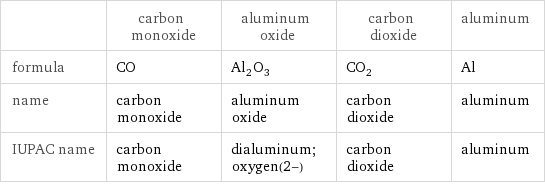
| carbon monoxide | aluminum oxide | carbon dioxide | aluminum formula | CO | Al_2O_3 | CO_2 | Al name | carbon monoxide | aluminum oxide | carbon dioxide | aluminum IUPAC name | carbon monoxide | dialuminum;oxygen(2-) | carbon dioxide | aluminum