Input interpretation
cobalt dichloride | sodium fluoride
Chemical names and formulas
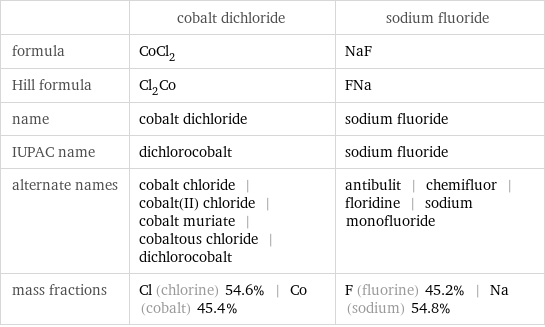
| cobalt dichloride | sodium fluoride formula | CoCl_2 | NaF Hill formula | Cl_2Co | FNa name | cobalt dichloride | sodium fluoride IUPAC name | dichlorocobalt | sodium fluoride alternate names | cobalt chloride | cobalt(II) chloride | cobalt muriate | cobaltous chloride | dichlorocobalt | antibulit | chemifluor | floridine | sodium monofluoride mass fractions | Cl (chlorine) 54.6% | Co (cobalt) 45.4% | F (fluorine) 45.2% | Na (sodium) 54.8%
Structure diagrams

Structure diagrams
3D structure
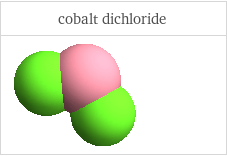
3D structure
Basic properties
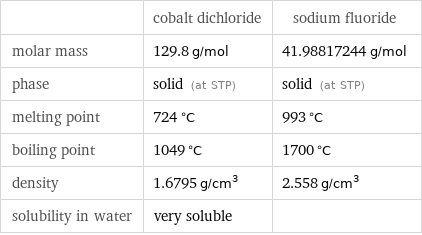
| cobalt dichloride | sodium fluoride molar mass | 129.8 g/mol | 41.98817244 g/mol phase | solid (at STP) | solid (at STP) melting point | 724 °C | 993 °C boiling point | 1049 °C | 1700 °C density | 1.6795 g/cm^3 | 2.558 g/cm^3 solubility in water | very soluble |
Units

Thermodynamic properties
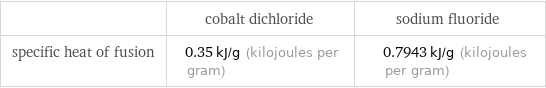
| cobalt dichloride | sodium fluoride specific heat of fusion | 0.35 kJ/g (kilojoules per gram) | 0.7943 kJ/g (kilojoules per gram)