Input interpretation
polar aprotic solvents | ebullioscopic constant K_b
Summary
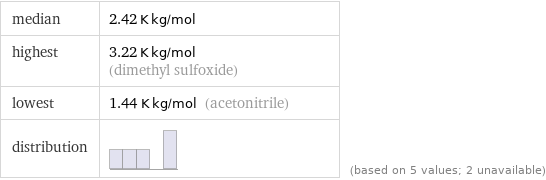
median | 2.42 K kg/mol highest | 3.22 K kg/mol (dimethyl sulfoxide) lowest | 1.44 K kg/mol (acetonitrile) distribution | | (based on 5 values; 2 unavailable)
Entities with missing values
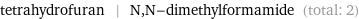
tetrahydrofuran | N, N-dimethylformamide (total: 2)
Ranked values
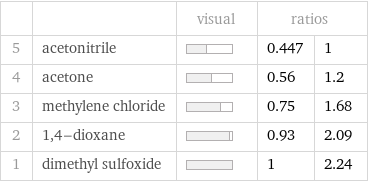
| | visual | ratios | 5 | acetonitrile | | 0.447 | 1 4 | acetone | | 0.56 | 1.2 3 | methylene chloride | | 0.75 | 1.68 2 | 1, 4-dioxane | | 0.93 | 2.09 1 | dimethyl sulfoxide | | 1 | 2.24
Ebullioscopic constant K_b rankings
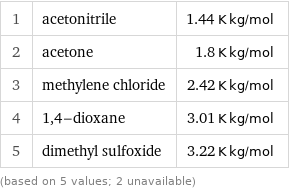
1 | acetonitrile | 1.44 K kg/mol 2 | acetone | 1.8 K kg/mol 3 | methylene chloride | 2.42 K kg/mol 4 | 1, 4-dioxane | 3.01 K kg/mol 5 | dimethyl sulfoxide | 3.22 K kg/mol (based on 5 values; 2 unavailable)
Unit conversions for median ebullioscopic constant K_b 2.42 K kg/mol
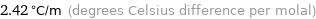
2.42 °C/m (degrees Celsius difference per molal)
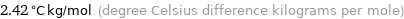
2.42 °C kg/mol (degree Celsius difference kilograms per mole)
Comparisons for median ebullioscopic constant K_b 2.42 K kg/mol

≈ 0.87 × boiling point elevation constant of cyclohexane ( ≈ 2.8 K kg/mol )
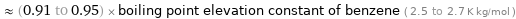
≈ (0.91 to 0.95) × boiling point elevation constant of benzene ( 2.5 to 2.7 K kg/mol )
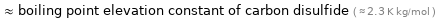
≈ boiling point elevation constant of carbon disulfide ( ≈ 2.3 K kg/mol )