Input interpretation
iridium | hydrogen peroxide
Chemical names and formulas
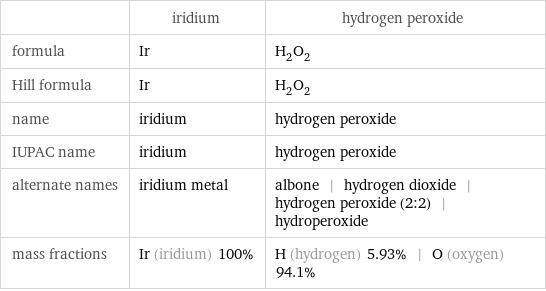
| iridium | hydrogen peroxide formula | Ir | H_2O_2 Hill formula | Ir | H_2O_2 name | iridium | hydrogen peroxide IUPAC name | iridium | hydrogen peroxide alternate names | iridium metal | albone | hydrogen dioxide | hydrogen peroxide (2:2) | hydroperoxide mass fractions | Ir (iridium) 100% | H (hydrogen) 5.93% | O (oxygen) 94.1%
Structure diagrams
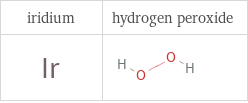
Structure diagrams
3D structure
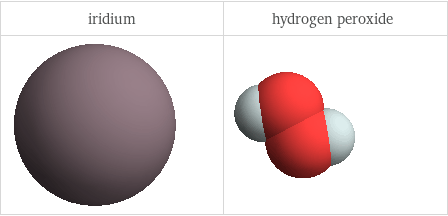
3D structure
Basic properties
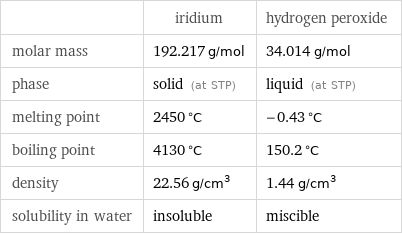
| iridium | hydrogen peroxide molar mass | 192.217 g/mol | 34.014 g/mol phase | solid (at STP) | liquid (at STP) melting point | 2450 °C | -0.43 °C boiling point | 4130 °C | 150.2 °C density | 22.56 g/cm^3 | 1.44 g/cm^3 solubility in water | insoluble | miscible
Units

Liquid properties
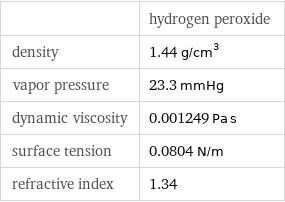
| hydrogen peroxide density | 1.44 g/cm^3 vapor pressure | 23.3 mmHg dynamic viscosity | 0.001249 Pa s surface tension | 0.0804 N/m refractive index | 1.34
Units
Thermodynamic properties
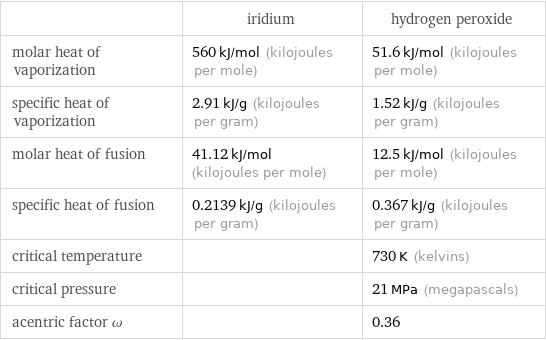
| iridium | hydrogen peroxide molar heat of vaporization | 560 kJ/mol (kilojoules per mole) | 51.6 kJ/mol (kilojoules per mole) specific heat of vaporization | 2.91 kJ/g (kilojoules per gram) | 1.52 kJ/g (kilojoules per gram) molar heat of fusion | 41.12 kJ/mol (kilojoules per mole) | 12.5 kJ/mol (kilojoules per mole) specific heat of fusion | 0.2139 kJ/g (kilojoules per gram) | 0.367 kJ/g (kilojoules per gram) critical temperature | | 730 K (kelvins) critical pressure | | 21 MPa (megapascals) acentric factor ω | | 0.36
Solid properties

| iridium density | 22.56 g/cm^3
Units
