Input interpretation
potassium permanganate | cobalt(II) basic carbonate
Chemical names and formulas
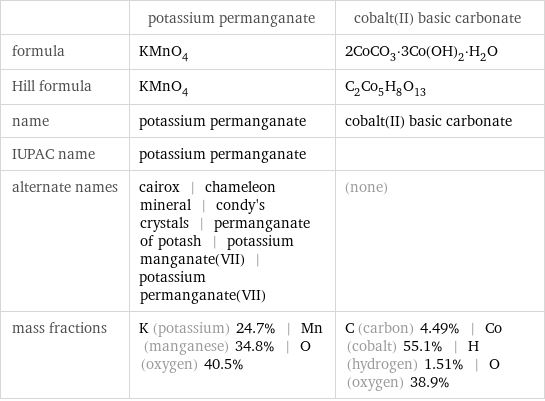
| potassium permanganate | cobalt(II) basic carbonate formula | KMnO_4 | 2CoCO_3·3Co(OH)_2·H_2O Hill formula | KMnO_4 | C_2Co_5H_8O_13 name | potassium permanganate | cobalt(II) basic carbonate IUPAC name | potassium permanganate | alternate names | cairox | chameleon mineral | condy's crystals | permanganate of potash | potassium manganate(VII) | potassium permanganate(VII) | (none) mass fractions | K (potassium) 24.7% | Mn (manganese) 34.8% | O (oxygen) 40.5% | C (carbon) 4.49% | Co (cobalt) 55.1% | H (hydrogen) 1.51% | O (oxygen) 38.9%
Structure diagrams
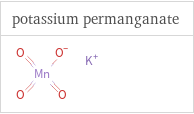
Structure diagrams
Basic properties
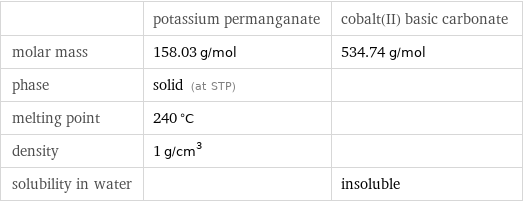
| potassium permanganate | cobalt(II) basic carbonate molar mass | 158.03 g/mol | 534.74 g/mol phase | solid (at STP) | melting point | 240 °C | density | 1 g/cm^3 | solubility in water | | insoluble
Units
