Input interpretation
potassium hydroxide
Chemical names and formulas

formula | KOH Hill formula | HKO name | potassium hydroxide alternate names | caustic potash | kaliumhydroxid | potassa | potassium hydrate mass fractions | H (hydrogen) 1.8% | K (potassium) 69.7% | O (oxygen) 28.5%
Structure diagram

Structure diagram
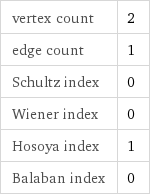
vertex count | 2 edge count | 1 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
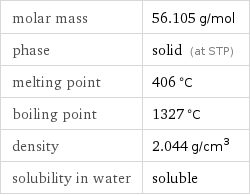
molar mass | 56.105 g/mol phase | solid (at STP) melting point | 406 °C boiling point | 1327 °C density | 2.044 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
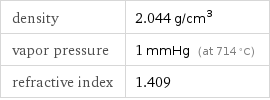
density | 2.044 g/cm^3 vapor pressure | 1 mmHg (at 714 °C) refractive index | 1.409
Units

Thermodynamic properties
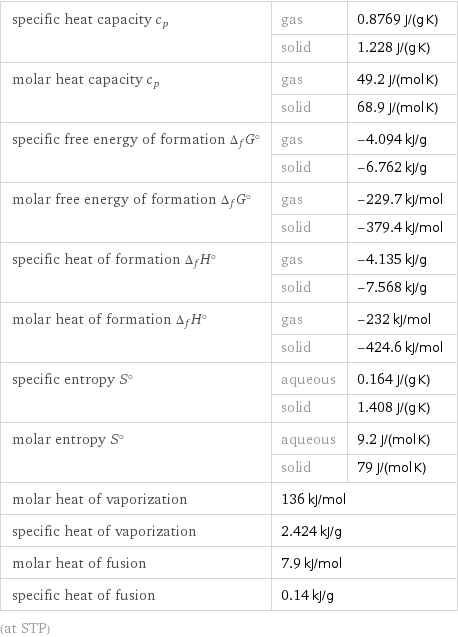
specific heat capacity c_p | gas | 0.8769 J/(g K) | solid | 1.228 J/(g K) molar heat capacity c_p | gas | 49.2 J/(mol K) | solid | 68.9 J/(mol K) specific free energy of formation Δ_fG° | gas | -4.094 kJ/g | solid | -6.762 kJ/g molar free energy of formation Δ_fG° | gas | -229.7 kJ/mol | solid | -379.4 kJ/mol specific heat of formation Δ_fH° | gas | -4.135 kJ/g | solid | -7.568 kJ/g molar heat of formation Δ_fH° | gas | -232 kJ/mol | solid | -424.6 kJ/mol specific entropy S° | aqueous | 0.164 J/(g K) | solid | 1.408 J/(g K) molar entropy S° | aqueous | 9.2 J/(mol K) | solid | 79 J/(mol K) molar heat of vaporization | 136 kJ/mol | specific heat of vaporization | 2.424 kJ/g | molar heat of fusion | 7.9 kJ/mol | specific heat of fusion | 0.14 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1310-58-3 PubChem CID number | 6093213 SMILES identifier | [OH-].[K+] InChI identifier | InChI=1/K.H2O/h;1H2/q+1;/p-1/fK.HO/h;1h/qm;-1 EU number | 215-181-3 Gmelin number | 19033 RTECS number | TT2100000](../image_source/03e605a0f52dfb062e90c919eeb3ae9f.png)
CAS number | 1310-58-3 PubChem CID number | 6093213 SMILES identifier | [OH-].[K+] InChI identifier | InChI=1/K.H2O/h;1H2/q+1;/p-1/fK.HO/h;1h/qm;-1 EU number | 215-181-3 Gmelin number | 19033 RTECS number | TT2100000
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1
Toxicity properties
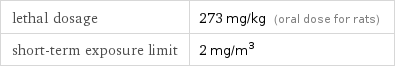
lethal dosage | 273 mg/kg (oral dose for rats) short-term exposure limit | 2 mg/m^3

probable lethal dose for man | 30 mL (milliliters) long-term exposure limit | 2 mg/m^3 (over 8 hours) RTECS classes | agricultural chemical and pesticide | mutagen | primary irritant
Ion equivalents

K^+ (potassium cation) | 1 (OH)^- (hydroxide anion) | 1