Input interpretation
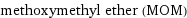
methoxymethyl ether (MOM)
Stability under oxidizing conditions
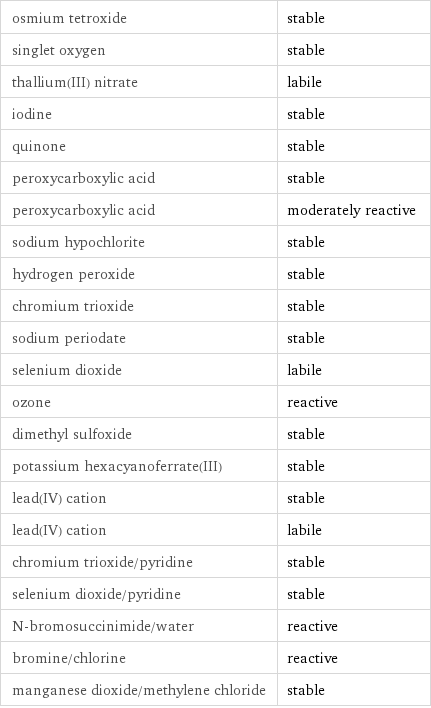
osmium tetroxide | stable singlet oxygen | stable thallium(III) nitrate | labile iodine | stable quinone | stable peroxycarboxylic acid | stable peroxycarboxylic acid | moderately reactive sodium hypochlorite | stable hydrogen peroxide | stable chromium trioxide | stable sodium periodate | stable selenium dioxide | labile ozone | reactive dimethyl sulfoxide | stable potassium hexacyanoferrate(III) | stable lead(IV) cation | stable lead(IV) cation | labile chromium trioxide/pyridine | stable selenium dioxide/pyridine | stable N-bromosuccinimide/water | reactive bromine/chlorine | reactive manganese dioxide/methylene chloride | stable
Stability under reducing conditions
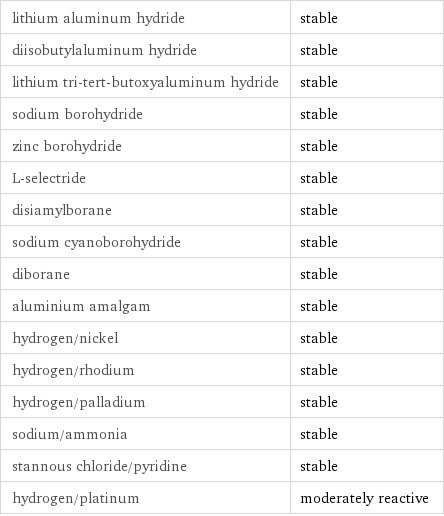
lithium aluminum hydride | stable diisobutylaluminum hydride | stable lithium tri-tert-butoxyaluminum hydride | stable sodium borohydride | stable zinc borohydride | stable L-selectride | stable disiamylborane | stable sodium cyanoborohydride | stable diborane | stable aluminium amalgam | stable hydrogen/nickel | stable hydrogen/rhodium | stable hydrogen/palladium | stable sodium/ammonia | stable stannous chloride/pyridine | stable hydrogen/platinum | moderately reactive
Stability under nucleophilic conditions
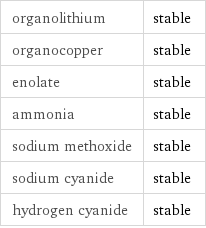
organolithium | stable organocopper | stable enolate | stable ammonia | stable sodium methoxide | stable sodium cyanide | stable hydrogen cyanide | stable
Stability under basic conditions
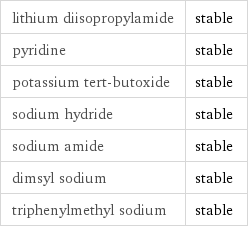
lithium diisopropylamide | stable pyridine | stable potassium tert-butoxide | stable sodium hydride | stable sodium amide | stable dimsyl sodium | stable triphenylmethyl sodium | stable
Stability under acidic conditions

lithium perchlorate/magnesium bromide | stable stannic chloride/boron trifluoride | labile copper(II) cation/pyridine | stable silver(I) cation | stable mercury(II) cation | stable
Stability under other conditions
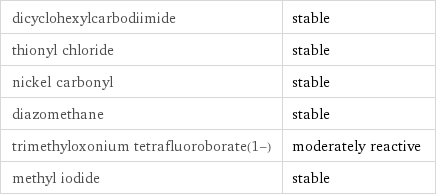
dicyclohexylcarbodiimide | stable thionyl chloride | stable nickel carbonyl | stable diazomethane | stable trimethyloxonium tetrafluoroborate(1-) | moderately reactive methyl iodide | stable
Stability under aqueous conditions
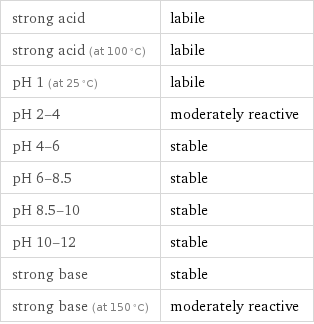
strong acid | labile strong acid (at 100 °C) | labile pH 1 (at 25 °C) | labile pH 2-4 | moderately reactive pH 4-6 | stable pH 6-8.5 | stable pH 8.5-10 | stable pH 10-12 | stable strong base | stable strong base (at 150 °C) | moderately reactive
Stability under thermal conditions

150 °C (degrees Celsius) | stable 250 °C (degrees Celsius) | stable 350 °C (degrees Celsius) | reactive
Stability under organometallic conditions
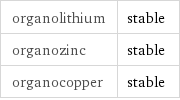
organolithium | stable organozinc | stable organocopper | stable