Input interpretation
tin (chemical element) | lead (chemical element) | sulfur (chemical element)
Periodic table location
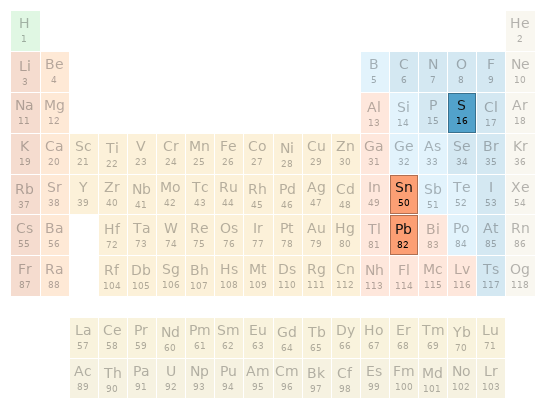
Periodic table location
Images

Images
Basic elemental properties
![| tin | lead | sulfur atomic symbol | Sn | Pb | S atomic number | 50 | 82 | 16 short electronic configuration | [Kr]5s^24d^105p^2 | [Xe]6s^24f^145d^106p^2 | [Ne]3s^23p^4 Aufbau diagram | 5p 4d 5s | 6p 5d 4f 6s | 3p 3s block | p | p | p group | 14 | 14 | 16 period | 5 | 6 | 3 atomic mass | 118.71 u | 207.2 u | 32.06 u half-life | (stable) | (stable) | (stable)](../image_source/bcb71c752a3375e5507f1d25357a5122.png)
| tin | lead | sulfur atomic symbol | Sn | Pb | S atomic number | 50 | 82 | 16 short electronic configuration | [Kr]5s^24d^105p^2 | [Xe]6s^24f^145d^106p^2 | [Ne]3s^23p^4 Aufbau diagram | 5p 4d 5s | 6p 5d 4f 6s | 3p 3s block | p | p | p group | 14 | 14 | 16 period | 5 | 6 | 3 atomic mass | 118.71 u | 207.2 u | 32.06 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
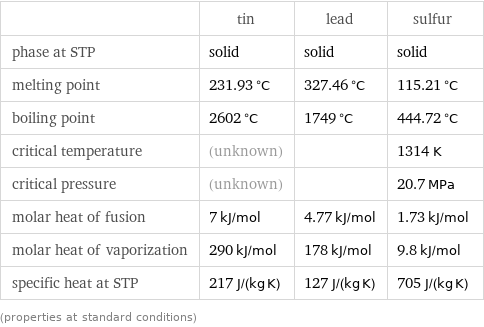
| tin | lead | sulfur phase at STP | solid | solid | solid melting point | 231.93 °C | 327.46 °C | 115.21 °C boiling point | 2602 °C | 1749 °C | 444.72 °C critical temperature | (unknown) | | 1314 K critical pressure | (unknown) | | 20.7 MPa molar heat of fusion | 7 kJ/mol | 4.77 kJ/mol | 1.73 kJ/mol molar heat of vaporization | 290 kJ/mol | 178 kJ/mol | 9.8 kJ/mol specific heat at STP | 217 J/(kg K) | 127 J/(kg K) | 705 J/(kg K) (properties at standard conditions)
Material properties
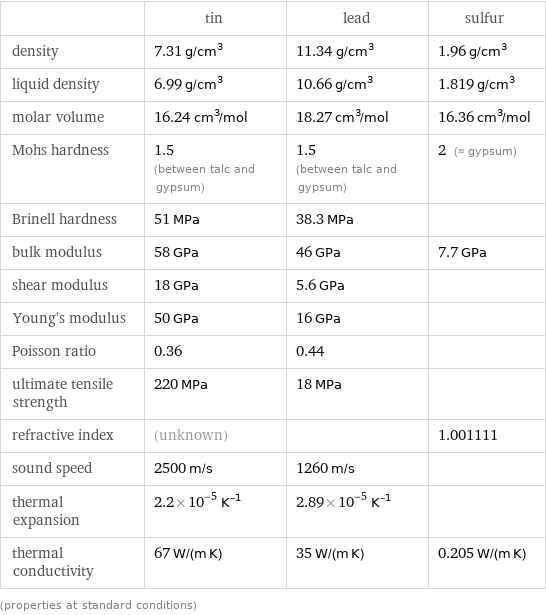
| tin | lead | sulfur density | 7.31 g/cm^3 | 11.34 g/cm^3 | 1.96 g/cm^3 liquid density | 6.99 g/cm^3 | 10.66 g/cm^3 | 1.819 g/cm^3 molar volume | 16.24 cm^3/mol | 18.27 cm^3/mol | 16.36 cm^3/mol Mohs hardness | 1.5 (between talc and gypsum) | 1.5 (between talc and gypsum) | 2 (≈ gypsum) Brinell hardness | 51 MPa | 38.3 MPa | bulk modulus | 58 GPa | 46 GPa | 7.7 GPa shear modulus | 18 GPa | 5.6 GPa | Young's modulus | 50 GPa | 16 GPa | Poisson ratio | 0.36 | 0.44 | ultimate tensile strength | 220 MPa | 18 MPa | refractive index | (unknown) | | 1.001111 sound speed | 2500 m/s | 1260 m/s | thermal expansion | 2.2×10^-5 K^(-1) | 2.89×10^-5 K^(-1) | thermal conductivity | 67 W/(m K) | 35 W/(m K) | 0.205 W/(m K) (properties at standard conditions)
Electromagnetic properties
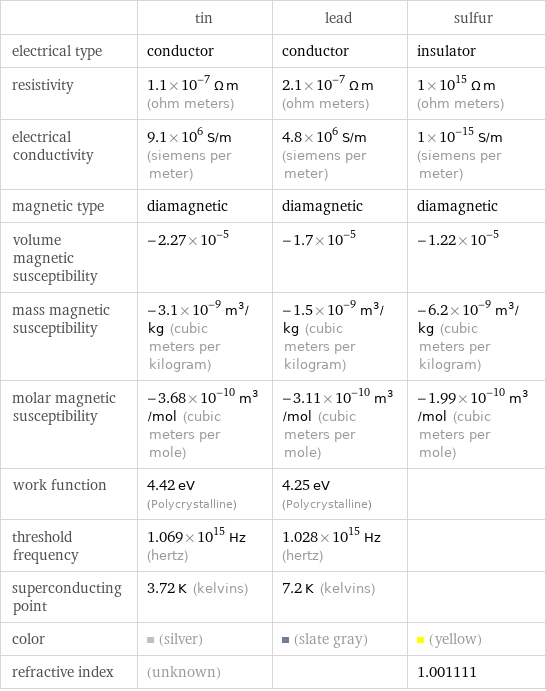
| tin | lead | sulfur electrical type | conductor | conductor | insulator resistivity | 1.1×10^-7 Ω m (ohm meters) | 2.1×10^-7 Ω m (ohm meters) | 1×10^15 Ω m (ohm meters) electrical conductivity | 9.1×10^6 S/m (siemens per meter) | 4.8×10^6 S/m (siemens per meter) | 1×10^-15 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -2.27×10^-5 | -1.7×10^-5 | -1.22×10^-5 mass magnetic susceptibility | -3.1×10^-9 m^3/kg (cubic meters per kilogram) | -1.5×10^-9 m^3/kg (cubic meters per kilogram) | -6.2×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -3.68×10^-10 m^3/mol (cubic meters per mole) | -3.11×10^-10 m^3/mol (cubic meters per mole) | -1.99×10^-10 m^3/mol (cubic meters per mole) work function | 4.42 eV (Polycrystalline) | 4.25 eV (Polycrystalline) | threshold frequency | 1.069×10^15 Hz (hertz) | 1.028×10^15 Hz (hertz) | superconducting point | 3.72 K (kelvins) | 7.2 K (kelvins) | color | (silver) | (slate gray) | (yellow) refractive index | (unknown) | | 1.001111
Reactivity
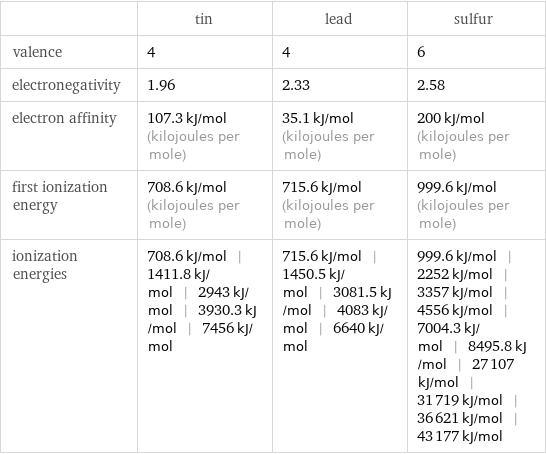
| tin | lead | sulfur valence | 4 | 4 | 6 electronegativity | 1.96 | 2.33 | 2.58 electron affinity | 107.3 kJ/mol (kilojoules per mole) | 35.1 kJ/mol (kilojoules per mole) | 200 kJ/mol (kilojoules per mole) first ionization energy | 708.6 kJ/mol (kilojoules per mole) | 715.6 kJ/mol (kilojoules per mole) | 999.6 kJ/mol (kilojoules per mole) ionization energies | 708.6 kJ/mol | 1411.8 kJ/mol | 2943 kJ/mol | 3930.3 kJ/mol | 7456 kJ/mol | 715.6 kJ/mol | 1450.5 kJ/mol | 3081.5 kJ/mol | 4083 kJ/mol | 6640 kJ/mol | 999.6 kJ/mol | 2252 kJ/mol | 3357 kJ/mol | 4556 kJ/mol | 7004.3 kJ/mol | 8495.8 kJ/mol | 27107 kJ/mol | 31719 kJ/mol | 36621 kJ/mol | 43177 kJ/mol
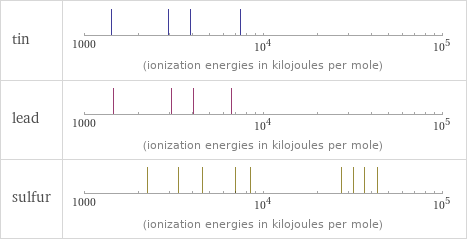
Reactivity
Atomic properties
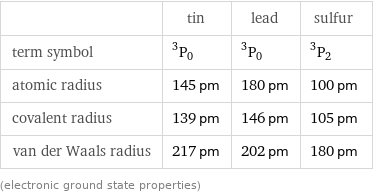
| tin | lead | sulfur term symbol | ^3P_0 | ^3P_0 | ^3P_2 atomic radius | 145 pm | 180 pm | 100 pm covalent radius | 139 pm | 146 pm | 105 pm van der Waals radius | 217 pm | 202 pm | 180 pm (electronic ground state properties)
Abundances
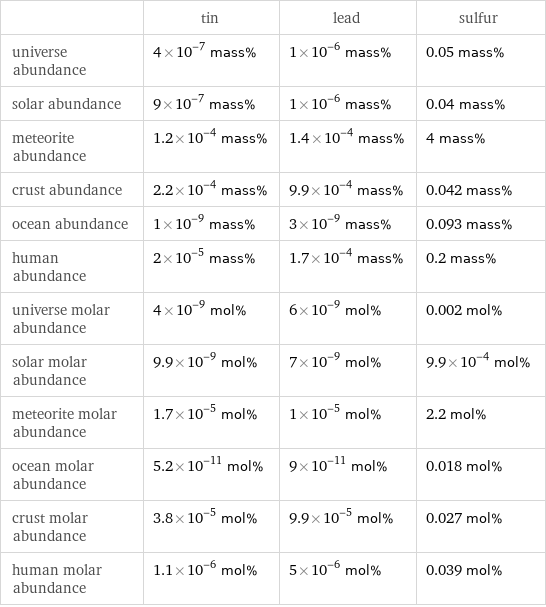
| tin | lead | sulfur universe abundance | 4×10^-7 mass% | 1×10^-6 mass% | 0.05 mass% solar abundance | 9×10^-7 mass% | 1×10^-6 mass% | 0.04 mass% meteorite abundance | 1.2×10^-4 mass% | 1.4×10^-4 mass% | 4 mass% crust abundance | 2.2×10^-4 mass% | 9.9×10^-4 mass% | 0.042 mass% ocean abundance | 1×10^-9 mass% | 3×10^-9 mass% | 0.093 mass% human abundance | 2×10^-5 mass% | 1.7×10^-4 mass% | 0.2 mass% universe molar abundance | 4×10^-9 mol% | 6×10^-9 mol% | 0.002 mol% solar molar abundance | 9.9×10^-9 mol% | 7×10^-9 mol% | 9.9×10^-4 mol% meteorite molar abundance | 1.7×10^-5 mol% | 1×10^-5 mol% | 2.2 mol% ocean molar abundance | 5.2×10^-11 mol% | 9×10^-11 mol% | 0.018 mol% crust molar abundance | 3.8×10^-5 mol% | 9.9×10^-5 mol% | 0.027 mol% human molar abundance | 1.1×10^-6 mol% | 5×10^-6 mol% | 0.039 mol%