Input interpretation

strong electrolytes | freezing point
Summary

median | 255 °C highest | 801 °C (sodium chloride) lowest | -114.17 °C (hydrogen chloride) distribution | | (based on 26 values; 6 unavailable)
Entities with missing values

lithium diethylamide | lithium diisopropylamide | strontium hydroxide | lithium bis(trimethylsilyl)amide | fluoroantimonic acid | magic acid (total: 6)
Distribution plots
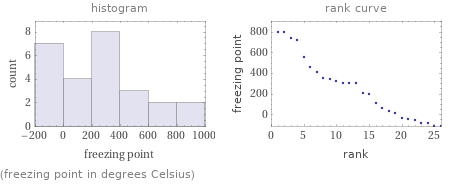
(freezing point in degrees Celsius)
Freezing point rankings
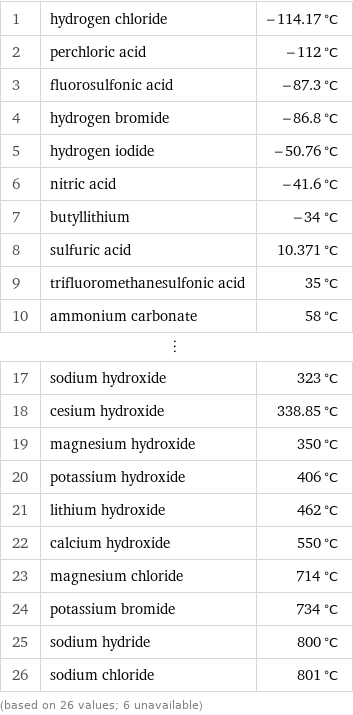
1 | hydrogen chloride | -114.17 °C 2 | perchloric acid | -112 °C 3 | fluorosulfonic acid | -87.3 °C 4 | hydrogen bromide | -86.8 °C 5 | hydrogen iodide | -50.76 °C 6 | nitric acid | -41.6 °C 7 | butyllithium | -34 °C 8 | sulfuric acid | 10.371 °C 9 | trifluoromethanesulfonic acid | 35 °C 10 | ammonium carbonate | 58 °C ⋮ | | 17 | sodium hydroxide | 323 °C 18 | cesium hydroxide | 338.85 °C 19 | magnesium hydroxide | 350 °C 20 | potassium hydroxide | 406 °C 21 | lithium hydroxide | 462 °C 22 | calcium hydroxide | 550 °C 23 | magnesium chloride | 714 °C 24 | potassium bromide | 734 °C 25 | sodium hydride | 800 °C 26 | sodium chloride | 801 °C (based on 26 values; 6 unavailable)
Unit conversions for median freezing point 255 °C
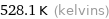
528.1 K (kelvins)
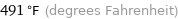
491 °F (degrees Fahrenheit)
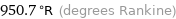
950.7 °R (degrees Rankine)

204 °Ré (degrees Réaumur)
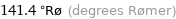
141.4 °Rø (degrees Rømer)
Comparison for median freezing point 255 °C
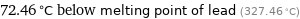
72.46 °C below melting point of lead (327.46 °C)

22.22 °C above autoignition temperature of book paper in Ray Bradbury's famous novel (451 °F)

(9 to 40) °C above autoignition temperature of paper (218 to 246 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 46 meV (millielectronvolts)
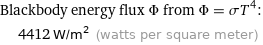
Blackbody energy flux Φ from Φ = σT^4: | 4412 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 1.4×10^-8 lx (lux)
Nearest corresponding gas marks for median freezing point 255 °C (degrees Celsius)
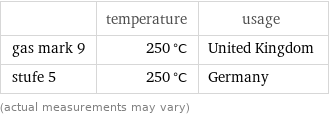
| temperature | usage gas mark 9 | 250 °C | United Kingdom stufe 5 | 250 °C | Germany (actual measurements may vary)