Input interpretation

Arrhenius acids | proton count
Summary
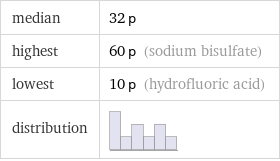
median | 32 p highest | 60 p (sodium bisulfate) lowest | 10 p (hydrofluoric acid) distribution |
Units

Proton count rankings
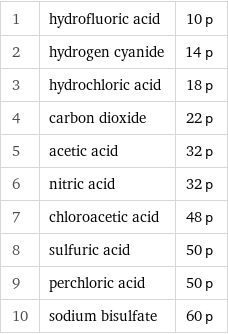
1 | hydrofluoric acid | 10 p 2 | hydrogen cyanide | 14 p 3 | hydrochloric acid | 18 p 4 | carbon dioxide | 22 p 5 | acetic acid | 32 p 6 | nitric acid | 32 p 7 | chloroacetic acid | 48 p 8 | sulfuric acid | 50 p 9 | perchloric acid | 50 p 10 | sodium bisulfate | 60 p
Corresponding quantity
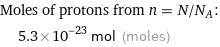
Moles of protons from n = N/N_A: | 5.3×10^-23 mol (moles)