Input interpretation

homonuclear diatomic molecules | molar magnetic susceptibility
Summary

median | -1.51×10^-10 m^3/mol highest | 4.334×10^-8 m^3/mol (oxygen) lowest | -1.1×10^-9 m^3/mol (iodine)
Units

Molar magnetic susceptibility rankings

1 | iodine | -1.1×10^-9 m^3/mol 2 | bromine | -7.087×10^-10 m^3/mol 3 | chlorine | -5.077×10^-10 m^3/mol 4 | nitrogen | -1.51×10^-10 m^3/mol 5 | fluorine | -1.21×10^-10 m^3/mol 6 | hydrogen | -5.014×10^-11 m^3/mol 7 | oxygen | 4.334×10^-8 m^3/mol
Unit conversions for median molar magnetic susceptibility -1.51×10^-10 m^3/mol
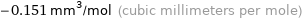
-0.151 mm^3/mol (cubic millimeters per mole)
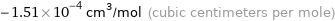
-1.51×10^-4 cm^3/mol (cubic centimeters per mole)