Input interpretation
difluorine dioxide | lead(II) oxalate
Chemical names and formulas
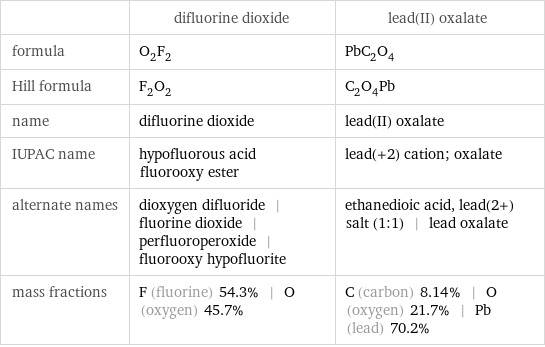
| difluorine dioxide | lead(II) oxalate formula | O_2F_2 | PbC_2O_4 Hill formula | F_2O_2 | C_2O_4Pb name | difluorine dioxide | lead(II) oxalate IUPAC name | hypofluorous acid fluorooxy ester | lead(+2) cation; oxalate alternate names | dioxygen difluoride | fluorine dioxide | perfluoroperoxide | fluorooxy hypofluorite | ethanedioic acid, lead(2+) salt (1:1) | lead oxalate mass fractions | F (fluorine) 54.3% | O (oxygen) 45.7% | C (carbon) 8.14% | O (oxygen) 21.7% | Pb (lead) 70.2%
Structure diagrams
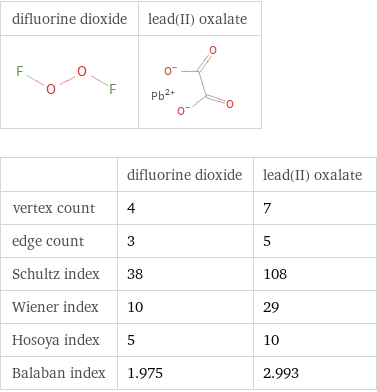
| difluorine dioxide | lead(II) oxalate vertex count | 4 | 7 edge count | 3 | 5 Schultz index | 38 | 108 Wiener index | 10 | 29 Hosoya index | 5 | 10 Balaban index | 1.975 | 2.993
3D structure
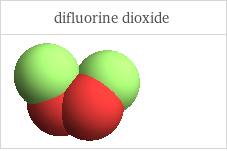
3D structure
Basic properties
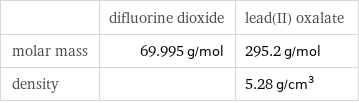
| difluorine dioxide | lead(II) oxalate molar mass | 69.995 g/mol | 295.2 g/mol density | | 5.28 g/cm^3
Units
