Input interpretation
chromic chloride | cobaltous nitrate hexahydrate
Chemical names and formulas
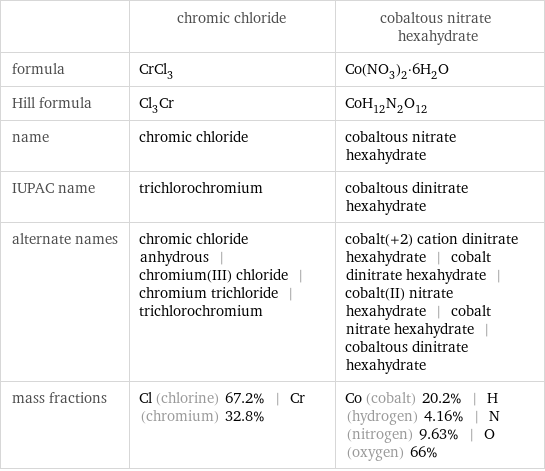
| chromic chloride | cobaltous nitrate hexahydrate formula | CrCl_3 | Co(NO_3)_2·6H_2O Hill formula | Cl_3Cr | CoH_12N_2O_12 name | chromic chloride | cobaltous nitrate hexahydrate IUPAC name | trichlorochromium | cobaltous dinitrate hexahydrate alternate names | chromic chloride anhydrous | chromium(III) chloride | chromium trichloride | trichlorochromium | cobalt(+2) cation dinitrate hexahydrate | cobalt dinitrate hexahydrate | cobalt(II) nitrate hexahydrate | cobalt nitrate hexahydrate | cobaltous dinitrate hexahydrate mass fractions | Cl (chlorine) 67.2% | Cr (chromium) 32.8% | Co (cobalt) 20.2% | H (hydrogen) 4.16% | N (nitrogen) 9.63% | O (oxygen) 66%
Structure diagrams
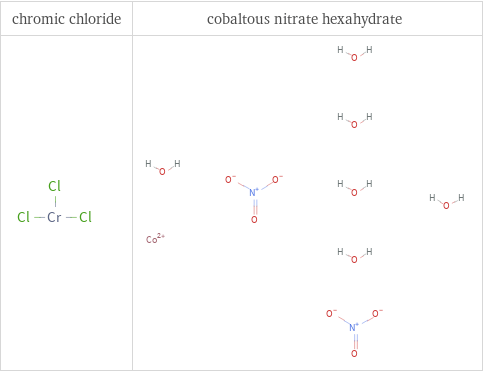
Structure diagrams
Basic properties
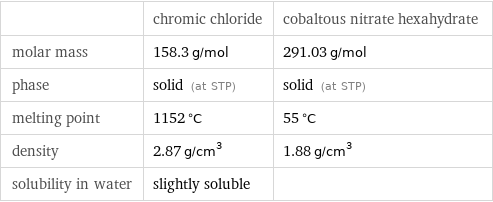
| chromic chloride | cobaltous nitrate hexahydrate molar mass | 158.3 g/mol | 291.03 g/mol phase | solid (at STP) | solid (at STP) melting point | 1152 °C | 55 °C density | 2.87 g/cm^3 | 1.88 g/cm^3 solubility in water | slightly soluble |
Units

Thermodynamic properties
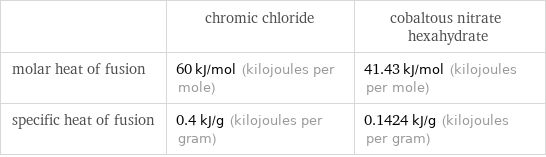
| chromic chloride | cobaltous nitrate hexahydrate molar heat of fusion | 60 kJ/mol (kilojoules per mole) | 41.43 kJ/mol (kilojoules per mole) specific heat of fusion | 0.4 kJ/g (kilojoules per gram) | 0.1424 kJ/g (kilojoules per gram)