Input interpretation
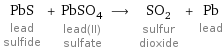
PbS lead sulfide + PbSO_4 lead(II) sulfate ⟶ SO_2 sulfur dioxide + Pb lead
Balanced equation

Balance the chemical equation algebraically: PbS + PbSO_4 ⟶ SO_2 + Pb Add stoichiometric coefficients, c_i, to the reactants and products: c_1 PbS + c_2 PbSO_4 ⟶ c_3 SO_2 + c_4 Pb Set the number of atoms in the reactants equal to the number of atoms in the products for Pb, S and O: Pb: | c_1 + c_2 = c_4 S: | c_1 + c_2 = c_3 O: | 4 c_2 = 2 c_3 Since the coefficients are relative quantities and underdetermined, choose a coefficient to set arbitrarily. To keep the coefficients small, the arbitrary value is ordinarily one. For instance, set c_1 = 1 and solve the system of equations for the remaining coefficients: c_1 = 1 c_2 = 1 c_3 = 2 c_4 = 2 Substitute the coefficients into the chemical reaction to obtain the balanced equation: Answer: | | PbS + PbSO_4 ⟶ 2 SO_2 + 2 Pb
Structures
+ ⟶ +
Names

lead sulfide + lead(II) sulfate ⟶ sulfur dioxide + lead
Reaction thermodynamics
Enthalpy
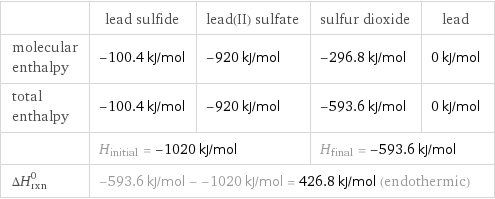
| lead sulfide | lead(II) sulfate | sulfur dioxide | lead molecular enthalpy | -100.4 kJ/mol | -920 kJ/mol | -296.8 kJ/mol | 0 kJ/mol total enthalpy | -100.4 kJ/mol | -920 kJ/mol | -593.6 kJ/mol | 0 kJ/mol | H_initial = -1020 kJ/mol | | H_final = -593.6 kJ/mol | ΔH_rxn^0 | -593.6 kJ/mol - -1020 kJ/mol = 426.8 kJ/mol (endothermic) | | |
Entropy
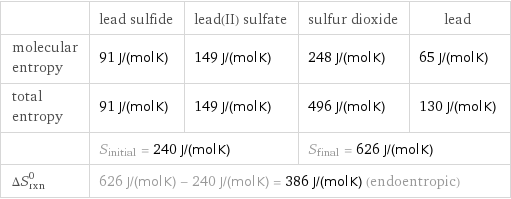
| lead sulfide | lead(II) sulfate | sulfur dioxide | lead molecular entropy | 91 J/(mol K) | 149 J/(mol K) | 248 J/(mol K) | 65 J/(mol K) total entropy | 91 J/(mol K) | 149 J/(mol K) | 496 J/(mol K) | 130 J/(mol K) | S_initial = 240 J/(mol K) | | S_final = 626 J/(mol K) | ΔS_rxn^0 | 626 J/(mol K) - 240 J/(mol K) = 386 J/(mol K) (endoentropic) | | |
Equilibrium constant
![Construct the equilibrium constant, K, expression for: PbS + PbSO_4 ⟶ SO_2 + Pb Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: PbS + PbSO_4 ⟶ 2 SO_2 + 2 Pb Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i PbS | 1 | -1 PbSO_4 | 1 | -1 SO_2 | 2 | 2 Pb | 2 | 2 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression PbS | 1 | -1 | ([PbS])^(-1) PbSO_4 | 1 | -1 | ([PbSO4])^(-1) SO_2 | 2 | 2 | ([SO2])^2 Pb | 2 | 2 | ([Pb])^2 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([PbS])^(-1) ([PbSO4])^(-1) ([SO2])^2 ([Pb])^2 = (([SO2])^2 ([Pb])^2)/([PbS] [PbSO4])](../image_source/458389e913a1dd80033323949d1d3154.png)
Construct the equilibrium constant, K, expression for: PbS + PbSO_4 ⟶ SO_2 + Pb Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the activity expression for each chemical species. • Use the activity expressions to build the equilibrium constant expression. Write the balanced chemical equation: PbS + PbSO_4 ⟶ 2 SO_2 + 2 Pb Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i PbS | 1 | -1 PbSO_4 | 1 | -1 SO_2 | 2 | 2 Pb | 2 | 2 Assemble the activity expressions accounting for the state of matter and ν_i: chemical species | c_i | ν_i | activity expression PbS | 1 | -1 | ([PbS])^(-1) PbSO_4 | 1 | -1 | ([PbSO4])^(-1) SO_2 | 2 | 2 | ([SO2])^2 Pb | 2 | 2 | ([Pb])^2 The equilibrium constant symbol in the concentration basis is: K_c Mulitply the activity expressions to arrive at the K_c expression: Answer: | | K_c = ([PbS])^(-1) ([PbSO4])^(-1) ([SO2])^2 ([Pb])^2 = (([SO2])^2 ([Pb])^2)/([PbS] [PbSO4])
Rate of reaction
![Construct the rate of reaction expression for: PbS + PbSO_4 ⟶ SO_2 + Pb Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: PbS + PbSO_4 ⟶ 2 SO_2 + 2 Pb Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i PbS | 1 | -1 PbSO_4 | 1 | -1 SO_2 | 2 | 2 Pb | 2 | 2 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term PbS | 1 | -1 | -(Δ[PbS])/(Δt) PbSO_4 | 1 | -1 | -(Δ[PbSO4])/(Δt) SO_2 | 2 | 2 | 1/2 (Δ[SO2])/(Δt) Pb | 2 | 2 | 1/2 (Δ[Pb])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -(Δ[PbS])/(Δt) = -(Δ[PbSO4])/(Δt) = 1/2 (Δ[SO2])/(Δt) = 1/2 (Δ[Pb])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)](../image_source/75a7878362bfa0f643909e6f9bf09c93.png)
Construct the rate of reaction expression for: PbS + PbSO_4 ⟶ SO_2 + Pb Plan: • Balance the chemical equation. • Determine the stoichiometric numbers. • Assemble the rate term for each chemical species. • Write the rate of reaction expression. Write the balanced chemical equation: PbS + PbSO_4 ⟶ 2 SO_2 + 2 Pb Assign stoichiometric numbers, ν_i, using the stoichiometric coefficients, c_i, from the balanced chemical equation in the following manner: ν_i = -c_i for reactants and ν_i = c_i for products: chemical species | c_i | ν_i PbS | 1 | -1 PbSO_4 | 1 | -1 SO_2 | 2 | 2 Pb | 2 | 2 The rate term for each chemical species, B_i, is 1/ν_i(Δ[B_i])/(Δt) where [B_i] is the amount concentration and t is time: chemical species | c_i | ν_i | rate term PbS | 1 | -1 | -(Δ[PbS])/(Δt) PbSO_4 | 1 | -1 | -(Δ[PbSO4])/(Δt) SO_2 | 2 | 2 | 1/2 (Δ[SO2])/(Δt) Pb | 2 | 2 | 1/2 (Δ[Pb])/(Δt) (for infinitesimal rate of change, replace Δ with d) Set the rate terms equal to each other to arrive at the rate expression: Answer: | | rate = -(Δ[PbS])/(Δt) = -(Δ[PbSO4])/(Δt) = 1/2 (Δ[SO2])/(Δt) = 1/2 (Δ[Pb])/(Δt) (assuming constant volume and no accumulation of intermediates or side products)
Chemical names and formulas
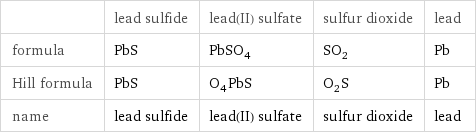
| lead sulfide | lead(II) sulfate | sulfur dioxide | lead formula | PbS | PbSO_4 | SO_2 | Pb Hill formula | PbS | O_4PbS | O_2S | Pb name | lead sulfide | lead(II) sulfate | sulfur dioxide | lead
Substance properties
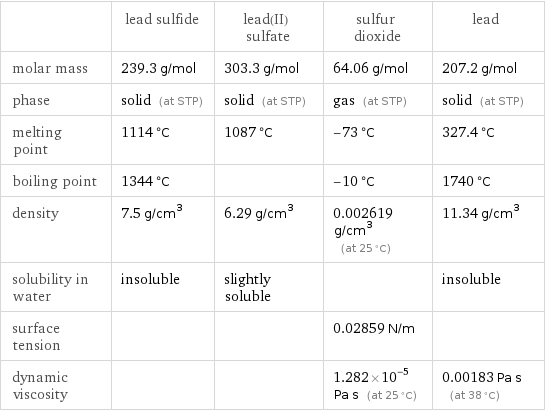
| lead sulfide | lead(II) sulfate | sulfur dioxide | lead molar mass | 239.3 g/mol | 303.3 g/mol | 64.06 g/mol | 207.2 g/mol phase | solid (at STP) | solid (at STP) | gas (at STP) | solid (at STP) melting point | 1114 °C | 1087 °C | -73 °C | 327.4 °C boiling point | 1344 °C | | -10 °C | 1740 °C density | 7.5 g/cm^3 | 6.29 g/cm^3 | 0.002619 g/cm^3 (at 25 °C) | 11.34 g/cm^3 solubility in water | insoluble | slightly soluble | | insoluble surface tension | | | 0.02859 N/m | dynamic viscosity | | | 1.282×10^-5 Pa s (at 25 °C) | 0.00183 Pa s (at 38 °C)
Units
