Input interpretation
non-polar covalent bonds
Members

hydrogen | fluorine | chlorine | bromine | iodine | nitrogen | oxygen (total: 7)
Structure
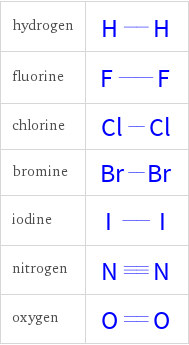
Structure
Basic properties
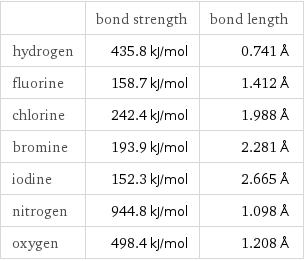
| bond strength | bond length hydrogen | 435.8 kJ/mol | 0.741 Å fluorine | 158.7 kJ/mol | 1.412 Å chlorine | 242.4 kJ/mol | 1.988 Å bromine | 193.9 kJ/mol | 2.281 Å iodine | 152.3 kJ/mol | 2.665 Å nitrogen | 944.8 kJ/mol | 1.098 Å oxygen | 498.4 kJ/mol | 1.208 Å
Units
