Input interpretation
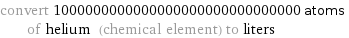
convert 1000000000000000000000000000000 atoms of helium (chemical element) to liters
Results
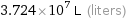
3.724×10^7 L (liters)
Possible intermediate steps
![Convert from atoms into liters: 1×10^30 atoms of He (helium) Divide by 1 atom/molecule to convert to molecules: (1×10^30 atoms)/1 × (1 molecule)/(1 atom) = 1×10^30 molecules Look up Avogadro's constant, N_A, to find the number of molecules in a mole: N_A = 6.022×10^23 molecules/mol Divide the number of moles by N_A to convert to moles: (1×10^30 molecules)/1 × (1 mol)/(6.022×10^23 molecules) = 1.661×10^6 mol The amount of substance, n, is: n = 1.661×10^6 mol The molar mass of He equals 4.002602 g/mol (grams per mole). The mass, m, in grams equals n multiplied by M: m = n M = (1.661×10^6 mol)/1 × (4.002602 g)/(1 mol) = 6.646477×10^6 g Convert from grams to cubic centimeters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter)[[2]] and the density 1.785×10^-4 g/cm^3 (grams per cubic centimeter) at STP of He: (6.646477×10^6 g)/1 × (1 cm^3)/(1.785×10^-4 g) = 3.724×10^10 cm^3 Convert to milliliters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter): (3.724×10^10 cm^3)/1 × (1 mL)/(1 cm^3) = 3.724×10^10 mL Convert to liters using the relation 1000 mL (milliliters) = 1 L (liter): Answer: | | (3.724×10^10 mL)/1 × (1 L)/(1000 mL) = 3.724×10^7 L](../image_source/4bc4695b93a891187981e2abb21e8d56.png)
Convert from atoms into liters: 1×10^30 atoms of He (helium) Divide by 1 atom/molecule to convert to molecules: (1×10^30 atoms)/1 × (1 molecule)/(1 atom) = 1×10^30 molecules Look up Avogadro's constant, N_A, to find the number of molecules in a mole: N_A = 6.022×10^23 molecules/mol Divide the number of moles by N_A to convert to moles: (1×10^30 molecules)/1 × (1 mol)/(6.022×10^23 molecules) = 1.661×10^6 mol The amount of substance, n, is: n = 1.661×10^6 mol The molar mass of He equals 4.002602 g/mol (grams per mole). The mass, m, in grams equals n multiplied by M: m = n M = (1.661×10^6 mol)/1 × (4.002602 g)/(1 mol) = 6.646477×10^6 g Convert from grams to cubic centimeters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter)[[2]] and the density 1.785×10^-4 g/cm^3 (grams per cubic centimeter) at STP of He: (6.646477×10^6 g)/1 × (1 cm^3)/(1.785×10^-4 g) = 3.724×10^10 cm^3 Convert to milliliters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter): (3.724×10^10 cm^3)/1 × (1 mL)/(1 cm^3) = 3.724×10^10 mL Convert to liters using the relation 1000 mL (milliliters) = 1 L (liter): Answer: | | (3.724×10^10 mL)/1 × (1 L)/(1000 mL) = 3.724×10^7 L
Unit conversions
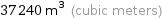
37240 m^3 (cubic meters)
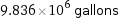
9.836×10^6 gallons
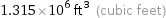
1.315×10^6 ft^3 (cubic feet)
Comparisons as volume
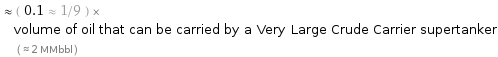
≈ ( 0.1 ≈ 1/9 ) × volume of oil that can be carried by a Very Large Crude Carrier supertanker ( ≈ 2 MMbbl )

≈ ( 0.2 ≈ 1/5 ) × volume of the Hindenburg Zeppelin ( ≈ 200000 m^3 )

≈ 0.63 × volume of steel used in the construction of the Three Gorges Dam ( ≈ 58900 m^3 )
Interpretations
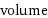
volume
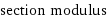
section modulus
Basic unit dimensions
![[length]^3](../image_source/b676a084adf842a81bc28fecb92c5196.png)
[length]^3
Corresponding quantities
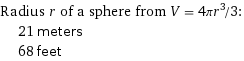
Radius r of a sphere from V = 4πr^3/3: | 21 meters | 68 feet
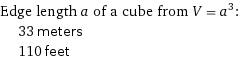
Edge length a of a cube from V = a^3: | 33 meters | 110 feet