Input interpretation
potassium chlorate
Chemical names and formulas

formula | KClO_3 Hill formula | ClKO_3 name | potassium chlorate alternate names | berthollet salt | chlorate of potash | potassium chlorate, solution mass fractions | Cl (chlorine) 28.9% | K (potassium) 31.9% | O (oxygen) 39.2%
Structure diagram
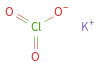
Structure diagram
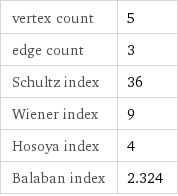
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties

molar mass | 122.5 g/mol phase | solid (at STP) melting point | 356 °C density | 2.34 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 2.34 g/cm^3 refractive index | 1.40835
Units

Thermodynamic properties
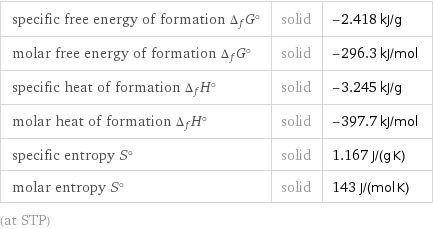
specific free energy of formation Δ_fG° | solid | -2.418 kJ/g molar free energy of formation Δ_fG° | solid | -296.3 kJ/mol specific heat of formation Δ_fH° | solid | -3.245 kJ/g molar heat of formation Δ_fH° | solid | -397.7 kJ/mol specific entropy S° | solid | 1.167 J/(g K) molar entropy S° | solid | 143 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 3811-04-9 PubChem CID number | 6426889 PubChem SID number | 24869084 SMILES identifier | [O-]Cl(=O)=O.[K+] InChI identifier | InChI=1/ClHO3.K/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fClO3.K/q-1;m RTECS number | FO0350000 MDL number | MFCD00011361](../image_source/e010fa6e0fd5665b46cf8ad8832de7a2.png)
CAS number | 3811-04-9 PubChem CID number | 6426889 PubChem SID number | 24869084 SMILES identifier | [O-]Cl(=O)=O.[K+] InChI identifier | InChI=1/ClHO3.K/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fClO3.K/q-1;m RTECS number | FO0350000 MDL number | MFCD00011361
NFPA label
NFPA label
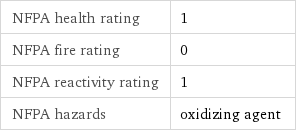
NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 1 NFPA hazards | oxidizing agent
Safety properties

flash point | 400 °C
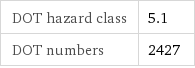
DOT hazard class | 5.1 DOT numbers | 2427
Toxicity properties
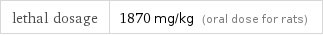
lethal dosage | 1870 mg/kg (oral dose for rats)

probable lethal dose for man | 600 mL (milliliters) RTECS classes | agricultural chemical and pesticide | human data
Ion equivalents

K^+ (potassium cation) | 1 (ClO_3)^- (chlorate anion) | 1