Input interpretation
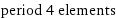
period 4 elements
Members

potassium | calcium | scandium | titanium | vanadium | chromium | manganese | iron | nickel | cobalt | copper | zinc | gallium | germanium | arsenic | selenium | bromine | krypton (total: 18)
Periodic table location
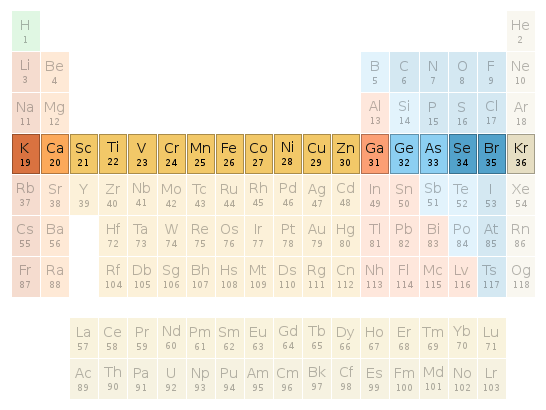
Periodic table location
Images
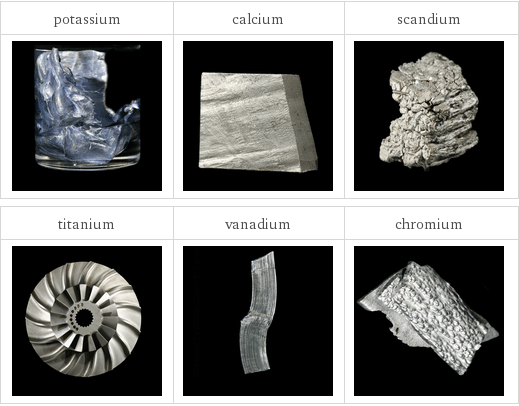
Images
Basic elemental properties
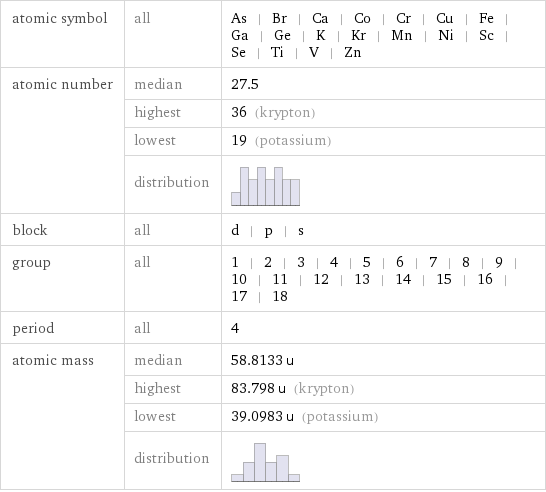
atomic symbol | all | As | Br | Ca | Co | Cr | Cu | Fe | Ga | Ge | K | Kr | Mn | Ni | Sc | Se | Ti | V | Zn atomic number | median | 27.5 | highest | 36 (krypton) | lowest | 19 (potassium) | distribution | block | all | d | p | s group | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 4 atomic mass | median | 58.8133 u | highest | 83.798 u (krypton) | lowest | 39.0983 u (potassium) | distribution |
Thermodynamic properties
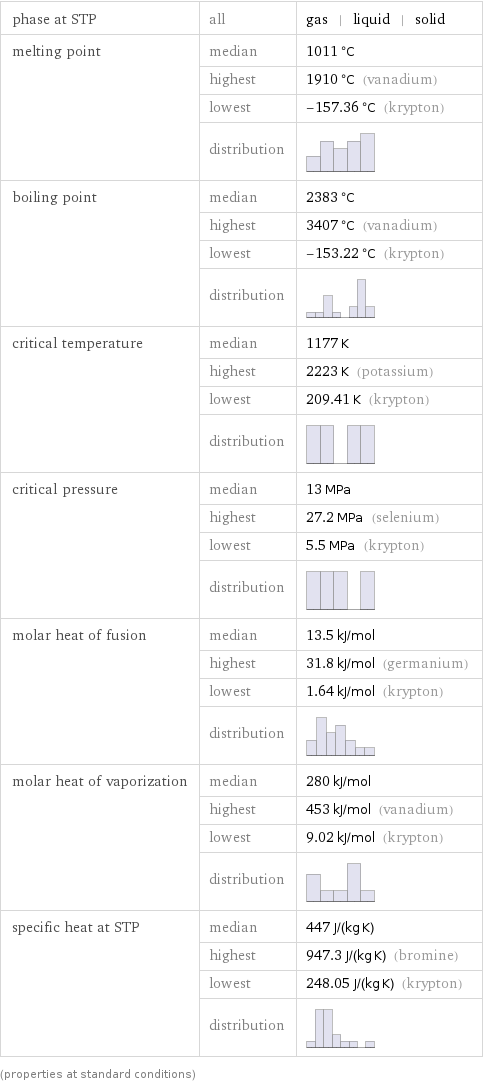
phase at STP | all | gas | liquid | solid melting point | median | 1011 °C | highest | 1910 °C (vanadium) | lowest | -157.36 °C (krypton) | distribution | boiling point | median | 2383 °C | highest | 3407 °C (vanadium) | lowest | -153.22 °C (krypton) | distribution | critical temperature | median | 1177 K | highest | 2223 K (potassium) | lowest | 209.41 K (krypton) | distribution | critical pressure | median | 13 MPa | highest | 27.2 MPa (selenium) | lowest | 5.5 MPa (krypton) | distribution | molar heat of fusion | median | 13.5 kJ/mol | highest | 31.8 kJ/mol (germanium) | lowest | 1.64 kJ/mol (krypton) | distribution | molar heat of vaporization | median | 280 kJ/mol | highest | 453 kJ/mol (vanadium) | lowest | 9.02 kJ/mol (krypton) | distribution | specific heat at STP | median | 447 J/(kg K) | highest | 947.3 J/(kg K) (bromine) | lowest | 248.05 J/(kg K) (krypton) | distribution | (properties at standard conditions)
Material properties
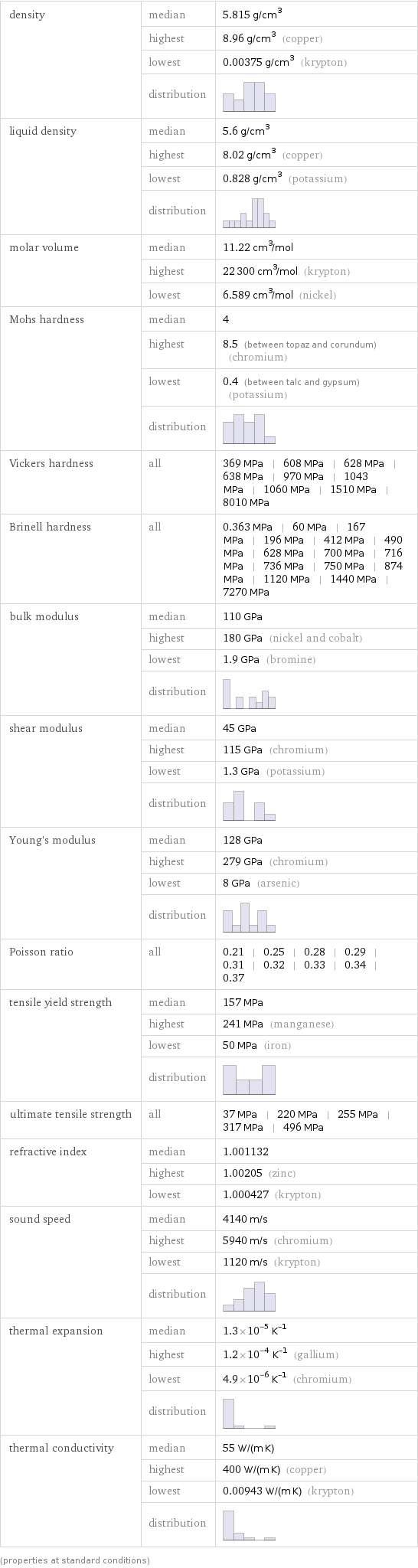
density | median | 5.815 g/cm^3 | highest | 8.96 g/cm^3 (copper) | lowest | 0.00375 g/cm^3 (krypton) | distribution | liquid density | median | 5.6 g/cm^3 | highest | 8.02 g/cm^3 (copper) | lowest | 0.828 g/cm^3 (potassium) | distribution | molar volume | median | 11.22 cm^3/mol | highest | 22300 cm^3/mol (krypton) | lowest | 6.589 cm^3/mol (nickel) Mohs hardness | median | 4 | highest | 8.5 (between topaz and corundum) (chromium) | lowest | 0.4 (between talc and gypsum) (potassium) | distribution | Vickers hardness | all | 369 MPa | 608 MPa | 628 MPa | 638 MPa | 970 MPa | 1043 MPa | 1060 MPa | 1510 MPa | 8010 MPa Brinell hardness | all | 0.363 MPa | 60 MPa | 167 MPa | 196 MPa | 412 MPa | 490 MPa | 628 MPa | 700 MPa | 716 MPa | 736 MPa | 750 MPa | 874 MPa | 1120 MPa | 1440 MPa | 7270 MPa bulk modulus | median | 110 GPa | highest | 180 GPa (nickel and cobalt) | lowest | 1.9 GPa (bromine) | distribution | shear modulus | median | 45 GPa | highest | 115 GPa (chromium) | lowest | 1.3 GPa (potassium) | distribution | Young's modulus | median | 128 GPa | highest | 279 GPa (chromium) | lowest | 8 GPa (arsenic) | distribution | Poisson ratio | all | 0.21 | 0.25 | 0.28 | 0.29 | 0.31 | 0.32 | 0.33 | 0.34 | 0.37 tensile yield strength | median | 157 MPa | highest | 241 MPa (manganese) | lowest | 50 MPa (iron) | distribution | ultimate tensile strength | all | 37 MPa | 220 MPa | 255 MPa | 317 MPa | 496 MPa refractive index | median | 1.001132 | highest | 1.00205 (zinc) | lowest | 1.000427 (krypton) sound speed | median | 4140 m/s | highest | 5940 m/s (chromium) | lowest | 1120 m/s (krypton) | distribution | thermal expansion | median | 1.3×10^-5 K^(-1) | highest | 1.2×10^-4 K^(-1) (gallium) | lowest | 4.9×10^-6 K^(-1) (chromium) | distribution | thermal conductivity | median | 55 W/(m K) | highest | 400 W/(m K) (copper) | lowest | 0.00943 W/(m K) (krypton) | distribution | (properties at standard conditions)
Electromagnetic properties

resistivity | median | 1.3×10^-7 Ω m (ohm meters) | highest | 1×10^10 Ω m (ohm meters) (bromine) | lowest | 1.7×10^-8 Ω m (ohm meters) (copper) electrical conductivity | median | 7.5×10^6 S/m (siemens per meter) | highest | 5.9×10^7 S/m (siemens per meter) (copper) | lowest | 1×10^-10 S/m (siemens per meter) (bromine) | distribution | volume magnetic susceptibility | median | -1.65×10^-8 | highest | 9.0387×10^-4 (manganese) | lowest | -2.23×10^-5 (arsenic) | distribution | mass magnetic susceptibility | median | -1.08×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.21×10^-7 m^3/kg (cubic meters per kilogram) (manganese) | lowest | -4.9×10^-9 m^3/kg (cubic meters per kilogram) (bromine) | distribution | molar magnetic susceptibility | median | -6.86×10^-11 m^3/mol (cubic meters per mole) | highest | 6.6475×10^-9 m^3/mol (cubic meters per mole) (manganese) | lowest | -7.83×10^-10 m^3/mol (cubic meters per mole) (bromine) | distribution | Curie point | median | 1043 K (kelvins) | highest | 1394 K (kelvins) (cobalt) | lowest | 631 K (kelvins) (nickel) | distribution | work function | all | 2.29 eV | 2.87 eV | 3.5 eV | 3.75 eV | 4.1 eV | 4.3 eV | 4.32 eV | 4.33 eV | 4.5 eV | 5 eV | 5.9 eV | (3.63 to 4.9) eV | (4.48 to 5.1) eV | (4.67 to 4.81) eV | (5.04 to 5.35) eV superconducting point | median | 0.85 K (kelvins) | highest | 5.4 K (kelvins) (vanadium) | lowest | 0.05 K (kelvins) (scandium) color | all |
Reactivity
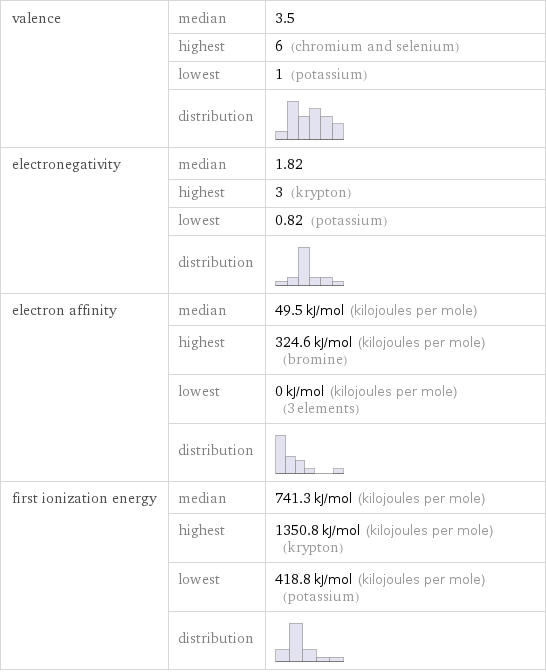
valence | median | 3.5 | highest | 6 (chromium and selenium) | lowest | 1 (potassium) | distribution | electronegativity | median | 1.82 | highest | 3 (krypton) | lowest | 0.82 (potassium) | distribution | electron affinity | median | 49.5 kJ/mol (kilojoules per mole) | highest | 324.6 kJ/mol (kilojoules per mole) (bromine) | lowest | 0 kJ/mol (kilojoules per mole) (3 elements) | distribution | first ionization energy | median | 741.3 kJ/mol (kilojoules per mole) | highest | 1350.8 kJ/mol (kilojoules per mole) (krypton) | lowest | 418.8 kJ/mol (kilojoules per mole) (potassium) | distribution |
Atomic properties
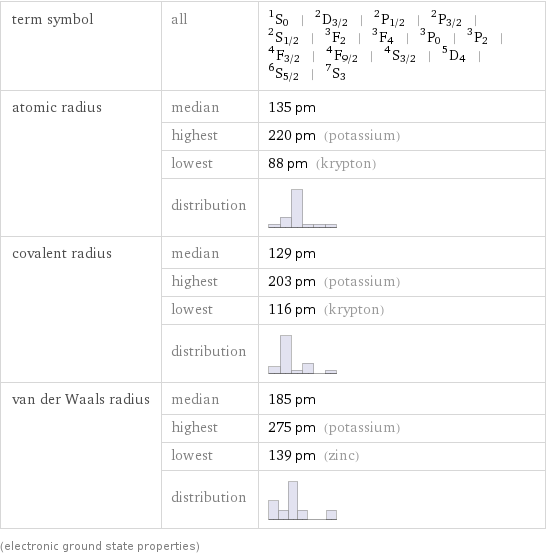
term symbol | all | ^1S_0 | ^2D_(3/2) | ^2P_(1/2) | ^2P_(3/2) | ^2S_(1/2) | ^3F_2 | ^3F_4 | ^3P_0 | ^3P_2 | ^4F_(3/2) | ^4F_(9/2) | ^4S_(3/2) | ^5D_4 | ^6S_(5/2) | ^7S_3 atomic radius | median | 135 pm | highest | 220 pm (potassium) | lowest | 88 pm (krypton) | distribution | covalent radius | median | 129 pm | highest | 203 pm (potassium) | lowest | 116 pm (krypton) | distribution | van der Waals radius | median | 185 pm | highest | 275 pm (potassium) | lowest | 139 pm (zinc) | distribution | (electronic ground state properties)
Abundances
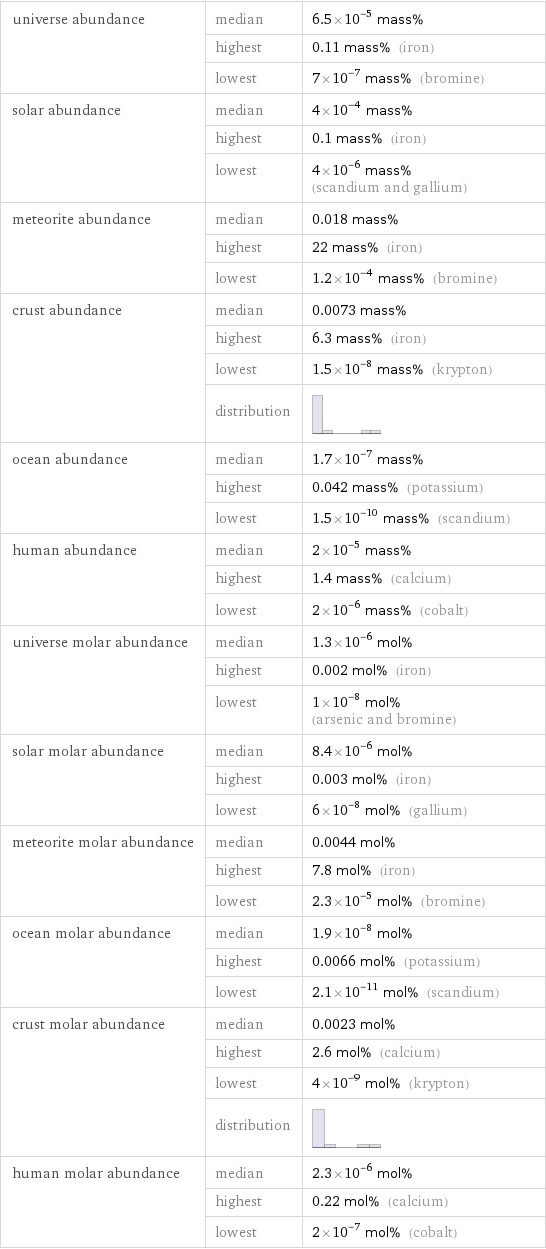
universe abundance | median | 6.5×10^-5 mass% | highest | 0.11 mass% (iron) | lowest | 7×10^-7 mass% (bromine) solar abundance | median | 4×10^-4 mass% | highest | 0.1 mass% (iron) | lowest | 4×10^-6 mass% (scandium and gallium) meteorite abundance | median | 0.018 mass% | highest | 22 mass% (iron) | lowest | 1.2×10^-4 mass% (bromine) crust abundance | median | 0.0073 mass% | highest | 6.3 mass% (iron) | lowest | 1.5×10^-8 mass% (krypton) | distribution | ocean abundance | median | 1.7×10^-7 mass% | highest | 0.042 mass% (potassium) | lowest | 1.5×10^-10 mass% (scandium) human abundance | median | 2×10^-5 mass% | highest | 1.4 mass% (calcium) | lowest | 2×10^-6 mass% (cobalt) universe molar abundance | median | 1.3×10^-6 mol% | highest | 0.002 mol% (iron) | lowest | 1×10^-8 mol% (arsenic and bromine) solar molar abundance | median | 8.4×10^-6 mol% | highest | 0.003 mol% (iron) | lowest | 6×10^-8 mol% (gallium) meteorite molar abundance | median | 0.0044 mol% | highest | 7.8 mol% (iron) | lowest | 2.3×10^-5 mol% (bromine) ocean molar abundance | median | 1.9×10^-8 mol% | highest | 0.0066 mol% (potassium) | lowest | 2.1×10^-11 mol% (scandium) crust molar abundance | median | 0.0023 mol% | highest | 2.6 mol% (calcium) | lowest | 4×10^-9 mol% (krypton) | distribution | human molar abundance | median | 2.3×10^-6 mol% | highest | 0.22 mol% (calcium) | lowest | 2×10^-7 mol% (cobalt)