Input interpretation

sodium nitrate | sodium
Chemical names and formulas
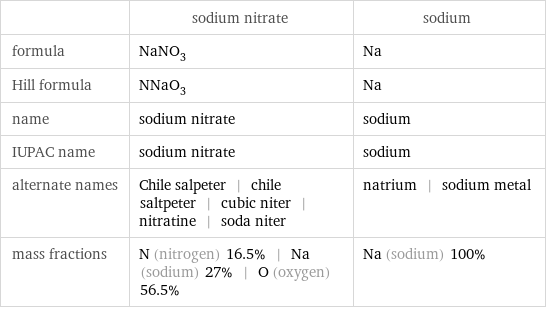
| sodium nitrate | sodium formula | NaNO_3 | Na Hill formula | NNaO_3 | Na name | sodium nitrate | sodium IUPAC name | sodium nitrate | sodium alternate names | Chile salpeter | chile saltpeter | cubic niter | nitratine | soda niter | natrium | sodium metal mass fractions | N (nitrogen) 16.5% | Na (sodium) 27% | O (oxygen) 56.5% | Na (sodium) 100%
Structure diagrams
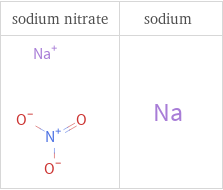
Structure diagrams
Basic properties
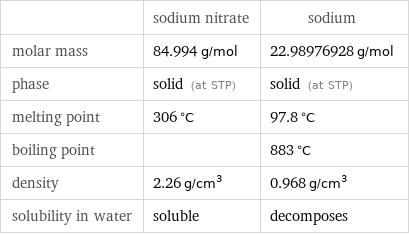
| sodium nitrate | sodium molar mass | 84.994 g/mol | 22.98976928 g/mol phase | solid (at STP) | solid (at STP) melting point | 306 °C | 97.8 °C boiling point | | 883 °C density | 2.26 g/cm^3 | 0.968 g/cm^3 solubility in water | soluble | decomposes
Units
