Input interpretation
lead(II) hydroxide | uranium(VI) oxide monohydrate
Chemical names and formulas
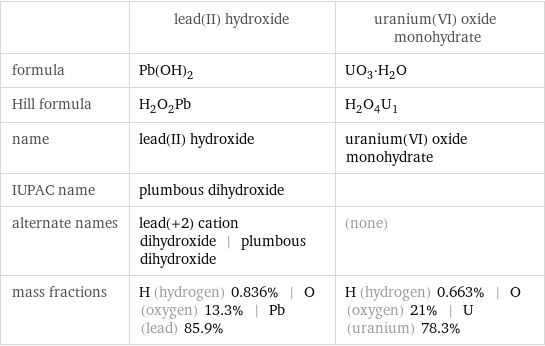
| lead(II) hydroxide | uranium(VI) oxide monohydrate formula | Pb(OH)_2 | UO_3·H_2O Hill formula | H_2O_2Pb | H_2O_4U_1 name | lead(II) hydroxide | uranium(VI) oxide monohydrate IUPAC name | plumbous dihydroxide | alternate names | lead(+2) cation dihydroxide | plumbous dihydroxide | (none) mass fractions | H (hydrogen) 0.836% | O (oxygen) 13.3% | Pb (lead) 85.9% | H (hydrogen) 0.663% | O (oxygen) 21% | U (uranium) 78.3%
Structure diagrams
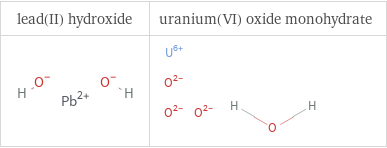
Structure diagrams
Basic properties
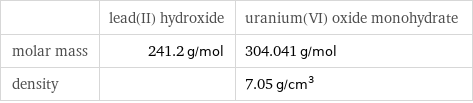
| lead(II) hydroxide | uranium(VI) oxide monohydrate molar mass | 241.2 g/mol | 304.041 g/mol density | | 7.05 g/cm^3
Units
