Input interpretation

interstellar molecules | relative molecular mass
Summary
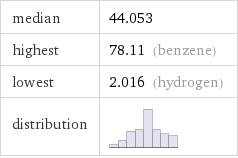
median | 44.053 highest | 78.11 (benzene) lowest | 2.016 (hydrogen) distribution |
Distribution plots
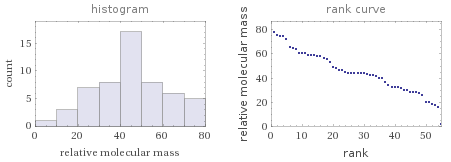
Relative molecular mass rankings
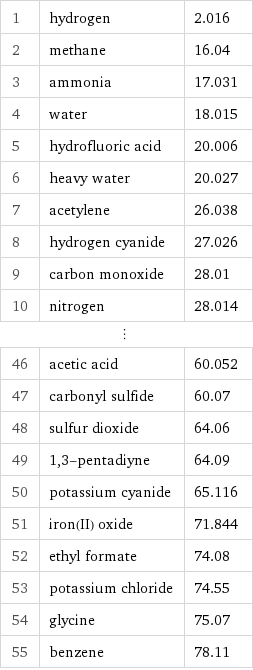
1 | hydrogen | 2.016 2 | methane | 16.04 3 | ammonia | 17.031 4 | water | 18.015 5 | hydrofluoric acid | 20.006 6 | heavy water | 20.027 7 | acetylene | 26.038 8 | hydrogen cyanide | 27.026 9 | carbon monoxide | 28.01 10 | nitrogen | 28.014 ⋮ | | 46 | acetic acid | 60.052 47 | carbonyl sulfide | 60.07 48 | sulfur dioxide | 64.06 49 | 1, 3-pentadiyne | 64.09 50 | potassium cyanide | 65.116 51 | iron(II) oxide | 71.844 52 | ethyl formate | 74.08 53 | potassium chloride | 74.55 54 | glycine | 75.07 55 | benzene | 78.11
Comparisons for median relative molecular mass 44.053
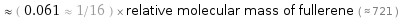
≈ ( 0.061 ≈ 1/16 ) × relative molecular mass of fullerene ( ≈ 721 )
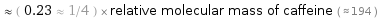
≈ ( 0.23 ≈ 1/4 ) × relative molecular mass of caffeine ( ≈ 194 )
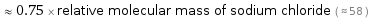
≈ 0.75 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
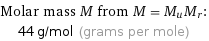
Molar mass M from M = M_uM_r: | 44 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 7.3×10^-23 grams | 7.3×10^-26 kg (kilograms)