Input interpretation
triflate anion | cyanide anion
Structure diagrams
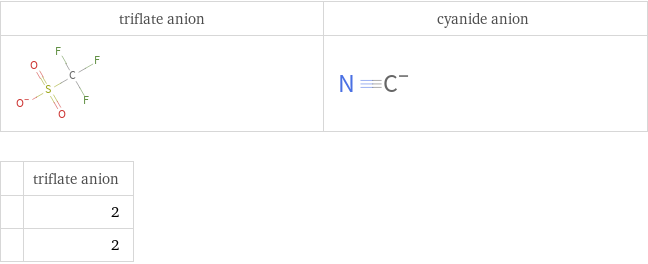
| triflate anion | 2 | 2
General properties
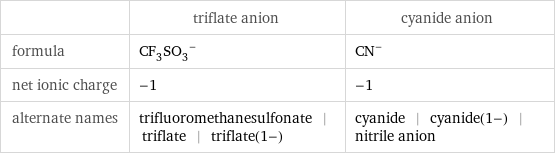
| triflate anion | cyanide anion formula | (CF_3SO_3)^- | (CN)^- net ionic charge | -1 | -1 alternate names | trifluoromethanesulfonate | triflate | triflate(1-) | cyanide | cyanide(1-) | nitrile anion
Ionic radii

| cyanide anion thermochemical radius | 191 pm
Units

Other properties
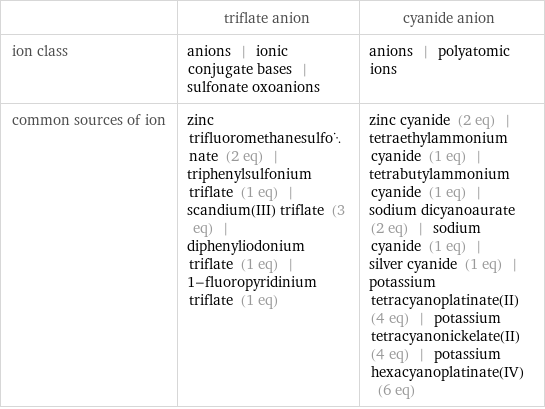
| triflate anion | cyanide anion ion class | anions | ionic conjugate bases | sulfonate oxoanions | anions | polyatomic ions common sources of ion | zinc trifluoromethanesulfonate (2 eq) | triphenylsulfonium triflate (1 eq) | scandium(III) triflate (3 eq) | diphenyliodonium triflate (1 eq) | 1-fluoropyridinium triflate (1 eq) | zinc cyanide (2 eq) | tetraethylammonium cyanide (1 eq) | tetrabutylammonium cyanide (1 eq) | sodium dicyanoaurate (2 eq) | sodium cyanide (1 eq) | silver cyanide (1 eq) | potassium tetracyanoplatinate(II) (4 eq) | potassium tetracyanonickelate(II) (4 eq) | potassium hexacyanoplatinate(IV) (6 eq)