Input interpretation

polar protic solvents | density
Summary
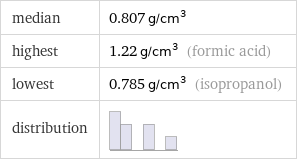
median | 0.807 g/cm^3 highest | 1.22 g/cm^3 (formic acid) lowest | 0.785 g/cm^3 (isopropanol) distribution |
Units

Ranked values
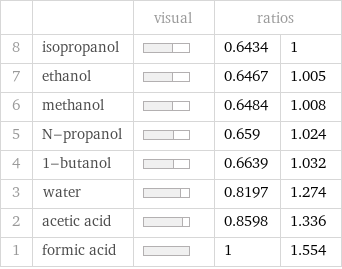
| | visual | ratios | 8 | isopropanol | | 0.6434 | 1 7 | ethanol | | 0.6467 | 1.005 6 | methanol | | 0.6484 | 1.008 5 | N-propanol | | 0.659 | 1.024 4 | 1-butanol | | 0.6639 | 1.032 3 | water | | 0.8197 | 1.274 2 | acetic acid | | 0.8598 | 1.336 1 | formic acid | | 1 | 1.554
Density rankings
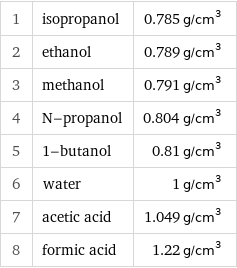
1 | isopropanol | 0.785 g/cm^3 2 | ethanol | 0.789 g/cm^3 3 | methanol | 0.791 g/cm^3 4 | N-propanol | 0.804 g/cm^3 5 | 1-butanol | 0.81 g/cm^3 6 | water | 1 g/cm^3 7 | acetic acid | 1.049 g/cm^3 8 | formic acid | 1.22 g/cm^3
Unit conversions for median density 0.807 g/cm^3
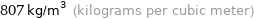
807 kg/m^3 (kilograms per cubic meter)
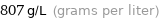
807 g/L (grams per liter)
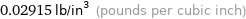
0.02915 lb/in^3 (pounds per cubic inch)
Comparisons for median density 0.807 g/cm^3

≈ 0.92 × diesel density ( ≈ 0.87 g/cm^3 )
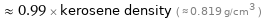
≈ 0.99 × kerosene density ( ≈ 0.819 g/cm^3 )

≈ ethanol density ( ≈ 790 kg/m^3 )