Input information
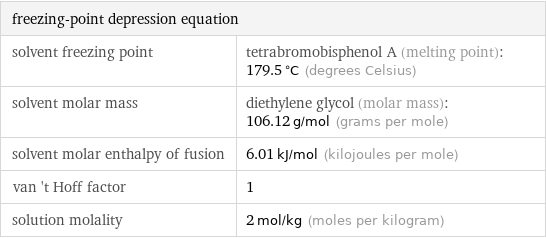
freezing-point depression equation | solvent freezing point | tetrabromobisphenol A (melting point): 179.5 °C (degrees Celsius) solvent molar mass | diethylene glycol (molar mass): 106.12 g/mol (grams per mole) solvent molar enthalpy of fusion | 6.01 kJ/mol (kilojoules per mole) van 't Hoff factor | 1 solution molality | 2 mol/kg (moles per kilogram)
Result
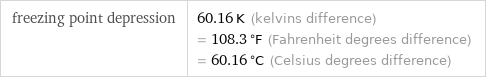
freezing point depression | 60.16 K (kelvins difference) = 108.3 °F (Fahrenheit degrees difference) = 60.16 °C (Celsius degrees difference)
Equation
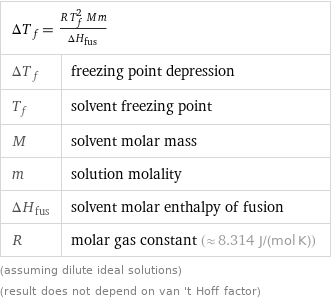
ΔT_f = (R T_f^2 M m)/ΔH_fus | ΔT_f | freezing point depression T_f | solvent freezing point M | solvent molar mass m | solution molality ΔH_fus | solvent molar enthalpy of fusion R | molar gas constant (≈ 8.314 J/(mol K)) (assuming dilute ideal solutions) (result does not depend on van 't Hoff factor)
Units
