Input interpretation

iron(II) oxalate dihydrate | tetraethylammonium hydroxide
Chemical names and formulas
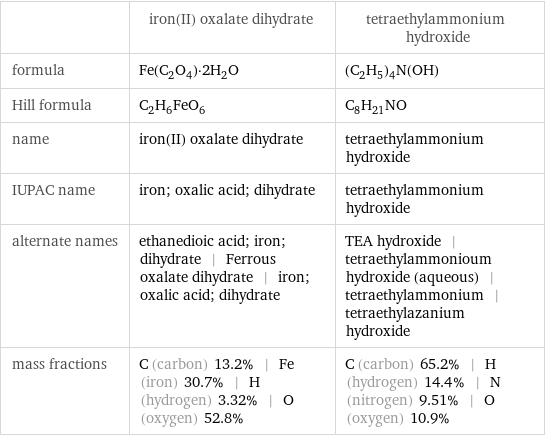
| iron(II) oxalate dihydrate | tetraethylammonium hydroxide formula | Fe(C_2O_4)·2H_2O | (C_2H_5)_4N(OH) Hill formula | C_2H_6FeO_6 | C_8H_21NO name | iron(II) oxalate dihydrate | tetraethylammonium hydroxide IUPAC name | iron; oxalic acid; dihydrate | tetraethylammonium hydroxide alternate names | ethanedioic acid; iron; dihydrate | Ferrous oxalate dihydrate | iron; oxalic acid; dihydrate | TEA hydroxide | tetraethylammonioum hydroxide (aqueous) | tetraethylammonium | tetraethylazanium hydroxide mass fractions | C (carbon) 13.2% | Fe (iron) 30.7% | H (hydrogen) 3.32% | O (oxygen) 52.8% | C (carbon) 65.2% | H (hydrogen) 14.4% | N (nitrogen) 9.51% | O (oxygen) 10.9%
Structure diagrams
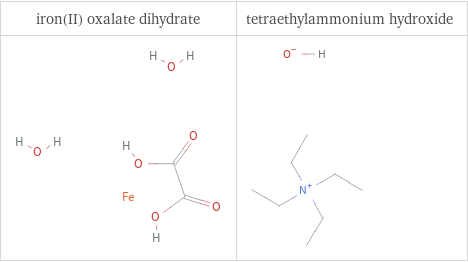
Structure diagrams
Basic properties
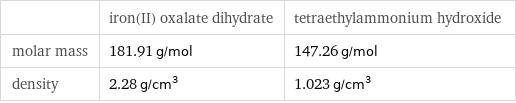
| iron(II) oxalate dihydrate | tetraethylammonium hydroxide molar mass | 181.91 g/mol | 147.26 g/mol density | 2.28 g/cm^3 | 1.023 g/cm^3
Units
