Input interpretation
Brønsted-Lowry acids | molar heat of vaporization
Summary
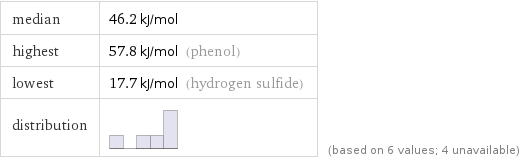
median | 46.2 kJ/mol highest | 57.8 kJ/mol (phenol) lowest | 17.7 kJ/mol (hydrogen sulfide) distribution | | (based on 6 values; 4 unavailable)
Entities with missing values
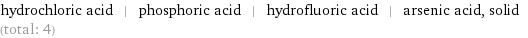
hydrochloric acid | phosphoric acid | hydrofluoric acid | arsenic acid, solid (total: 4)
Molar heat of vaporization rankings
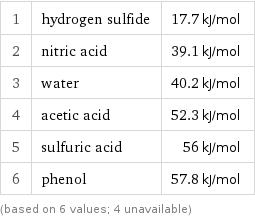
1 | hydrogen sulfide | 17.7 kJ/mol 2 | nitric acid | 39.1 kJ/mol 3 | water | 40.2 kJ/mol 4 | acetic acid | 52.3 kJ/mol 5 | sulfuric acid | 56 kJ/mol 6 | phenol | 57.8 kJ/mol (based on 6 values; 4 unavailable)
Unit conversions for median molar heat of vaporization 46.2 kJ/mol
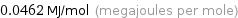
0.0462 MJ/mol (megajoules per mole)
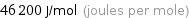
46200 J/mol (joules per mole)
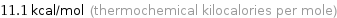
11.1 kcal/mol (thermochemical kilocalories per mole)
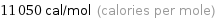
11050 cal/mol (calories per mole)

0.479 eV (molar electronvolts)