Input interpretation
halogenated compounds
Members

1, 1, 2, 2-tetrafluoroethane | 1-chloro-1-propene | acetyl fluoride | chlorocyclopentane | methyl chloride | cupric fluoride | fluorocyclohexane | fluoromethane | hydrofluoric acid | hydrogen bromide | hydrogen chloride | iodoacetonitrile | lithium bromide | lithium chloride | potassium chloride | potassium iodide | rubidium fluoride | sodium bromide | sodium fluoride | zinc fluoride (total: 20)
Basic properties
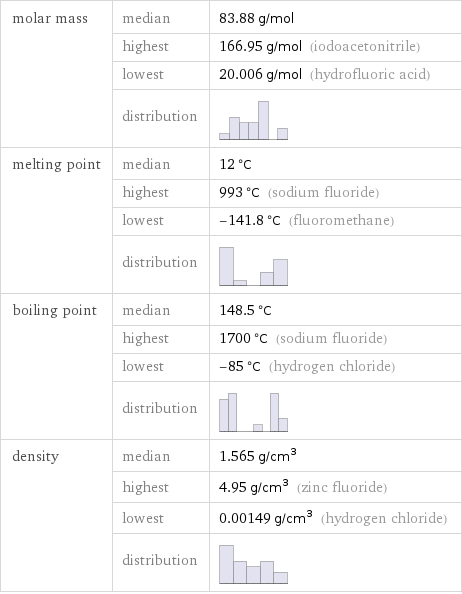
molar mass | median | 83.88 g/mol | highest | 166.95 g/mol (iodoacetonitrile) | lowest | 20.006 g/mol (hydrofluoric acid) | distribution | melting point | median | 12 °C | highest | 993 °C (sodium fluoride) | lowest | -141.8 °C (fluoromethane) | distribution | boiling point | median | 148.5 °C | highest | 1700 °C (sodium fluoride) | lowest | -85 °C (hydrogen chloride) | distribution | density | median | 1.565 g/cm^3 | highest | 4.95 g/cm^3 (zinc fluoride) | lowest | 0.00149 g/cm^3 (hydrogen chloride) | distribution |
Units
