Input interpretation
actinides
Members

actinium | thorium | protactinium | uranium | neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium (total: 15)
Periodic table location
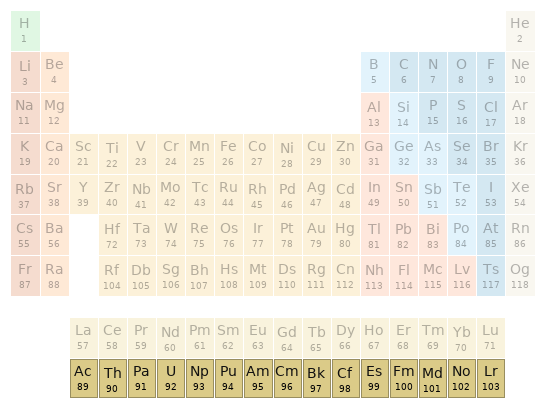
Periodic table location
Images
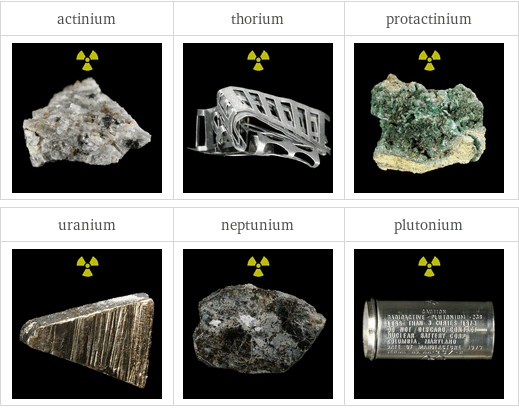
Images
Basic elemental properties
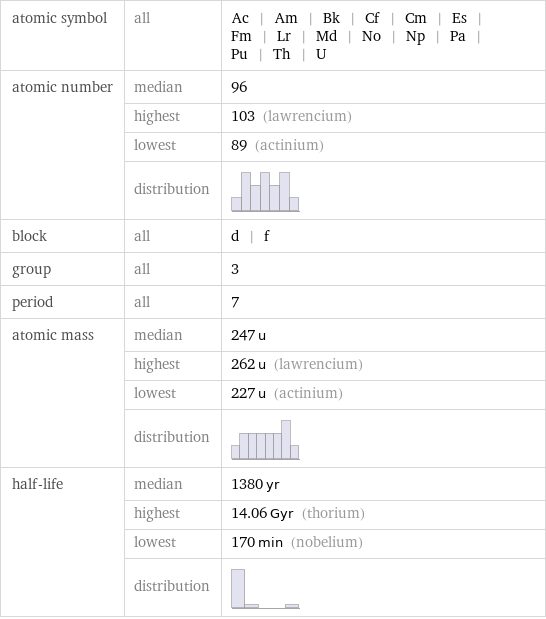
atomic symbol | all | Ac | Am | Bk | Cf | Cm | Es | Fm | Lr | Md | No | Np | Pa | Pu | Th | U atomic number | median | 96 | highest | 103 (lawrencium) | lowest | 89 (actinium) | distribution | block | all | d | f group | all | 3 period | all | 7 atomic mass | median | 247 u | highest | 262 u (lawrencium) | lowest | 227 u (actinium) | distribution | half-life | median | 1380 yr | highest | 14.06 Gyr (thorium) | lowest | 170 min (nobelium) | distribution |
Thermodynamic properties
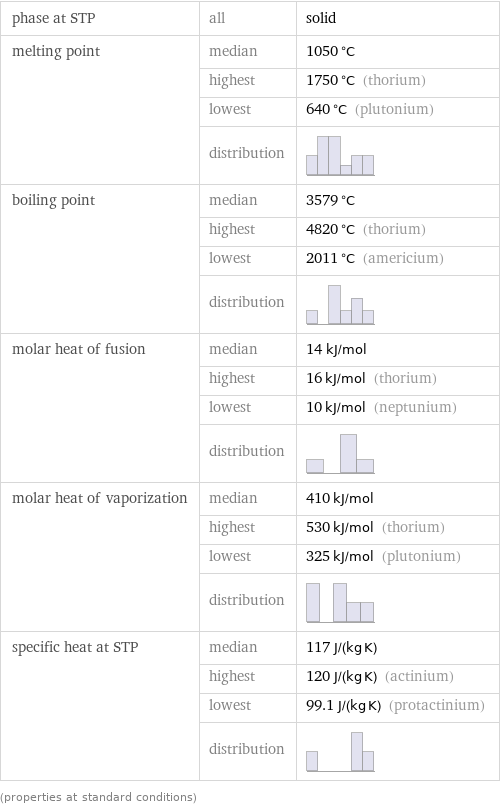
phase at STP | all | solid melting point | median | 1050 °C | highest | 1750 °C (thorium) | lowest | 640 °C (plutonium) | distribution | boiling point | median | 3579 °C | highest | 4820 °C (thorium) | lowest | 2011 °C (americium) | distribution | molar heat of fusion | median | 14 kJ/mol | highest | 16 kJ/mol (thorium) | lowest | 10 kJ/mol (neptunium) | distribution | molar heat of vaporization | median | 410 kJ/mol | highest | 530 kJ/mol (thorium) | lowest | 325 kJ/mol (plutonium) | distribution | specific heat at STP | median | 117 J/(kg K) | highest | 120 J/(kg K) (actinium) | lowest | 99.1 J/(kg K) (protactinium) | distribution | (properties at standard conditions)
Material properties
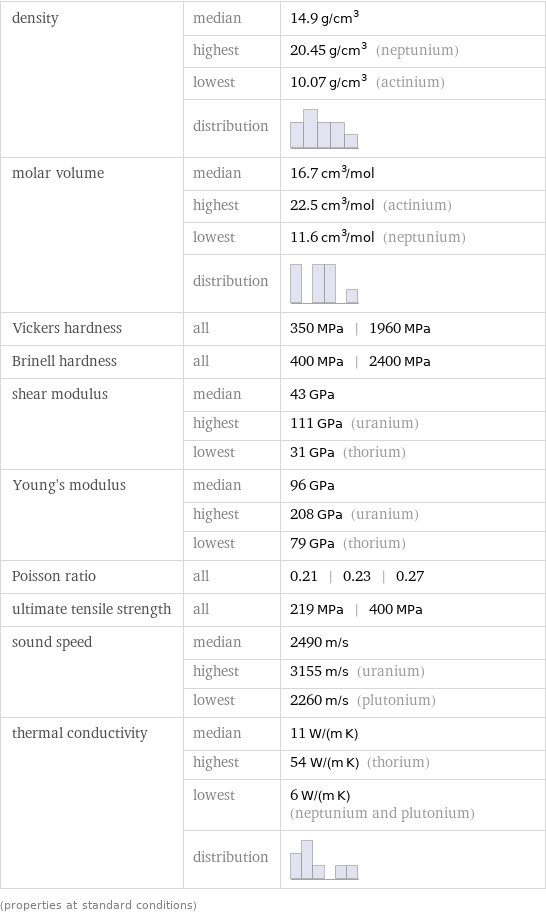
density | median | 14.9 g/cm^3 | highest | 20.45 g/cm^3 (neptunium) | lowest | 10.07 g/cm^3 (actinium) | distribution | molar volume | median | 16.7 cm^3/mol | highest | 22.5 cm^3/mol (actinium) | lowest | 11.6 cm^3/mol (neptunium) | distribution | Vickers hardness | all | 350 MPa | 1960 MPa Brinell hardness | all | 400 MPa | 2400 MPa shear modulus | median | 43 GPa | highest | 111 GPa (uranium) | lowest | 31 GPa (thorium) Young's modulus | median | 96 GPa | highest | 208 GPa (uranium) | lowest | 79 GPa (thorium) Poisson ratio | all | 0.21 | 0.23 | 0.27 ultimate tensile strength | all | 219 MPa | 400 MPa sound speed | median | 2490 m/s | highest | 3155 m/s (uranium) | lowest | 2260 m/s (plutonium) thermal conductivity | median | 11 W/(m K) | highest | 54 W/(m K) (thorium) | lowest | 6 W/(m K) (neptunium and plutonium) | distribution | (properties at standard conditions)
Electromagnetic properties
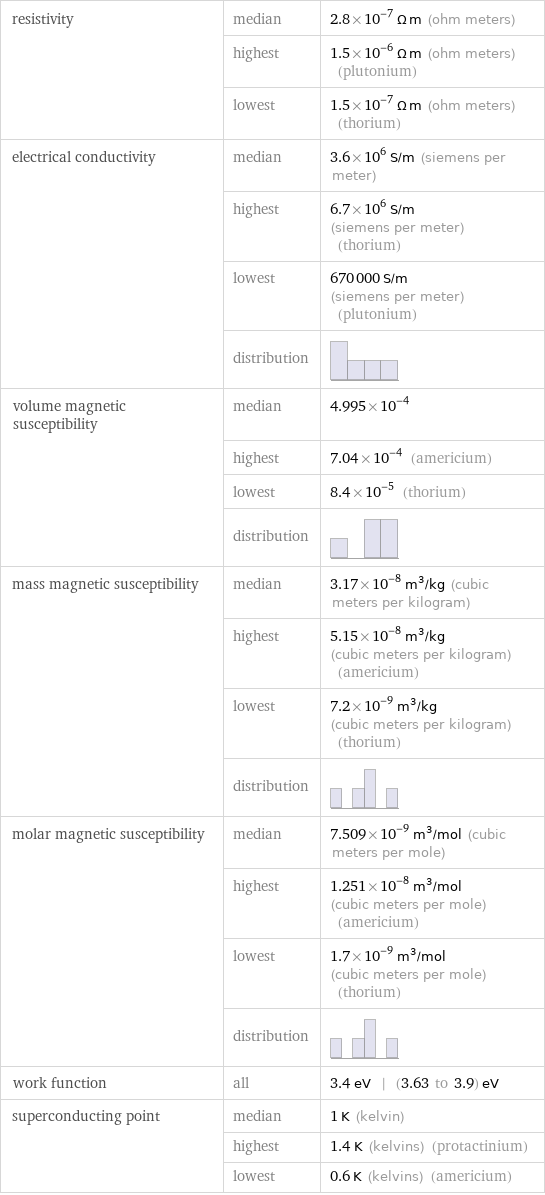
resistivity | median | 2.8×10^-7 Ω m (ohm meters) | highest | 1.5×10^-6 Ω m (ohm meters) (plutonium) | lowest | 1.5×10^-7 Ω m (ohm meters) (thorium) electrical conductivity | median | 3.6×10^6 S/m (siemens per meter) | highest | 6.7×10^6 S/m (siemens per meter) (thorium) | lowest | 670000 S/m (siemens per meter) (plutonium) | distribution | volume magnetic susceptibility | median | 4.995×10^-4 | highest | 7.04×10^-4 (americium) | lowest | 8.4×10^-5 (thorium) | distribution | mass magnetic susceptibility | median | 3.17×10^-8 m^3/kg (cubic meters per kilogram) | highest | 5.15×10^-8 m^3/kg (cubic meters per kilogram) (americium) | lowest | 7.2×10^-9 m^3/kg (cubic meters per kilogram) (thorium) | distribution | molar magnetic susceptibility | median | 7.509×10^-9 m^3/mol (cubic meters per mole) | highest | 1.251×10^-8 m^3/mol (cubic meters per mole) (americium) | lowest | 1.7×10^-9 m^3/mol (cubic meters per mole) (thorium) | distribution | work function | all | 3.4 eV | (3.63 to 3.9) eV superconducting point | median | 1 K (kelvin) | highest | 1.4 K (kelvins) (protactinium) | lowest | 0.6 K (kelvins) (americium)
Reactivity
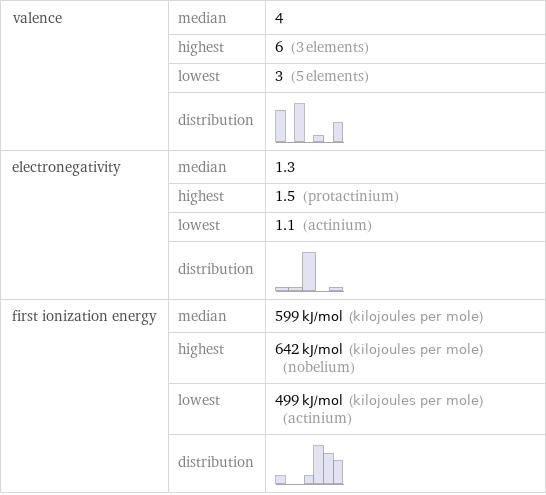
valence | median | 4 | highest | 6 (3 elements) | lowest | 3 (5 elements) | distribution | electronegativity | median | 1.3 | highest | 1.5 (protactinium) | lowest | 1.1 (actinium) | distribution | first ionization energy | median | 599 kJ/mol (kilojoules per mole) | highest | 642 kJ/mol (kilojoules per mole) (nobelium) | lowest | 499 kJ/mol (kilojoules per mole) (actinium) | distribution |
Atomic properties
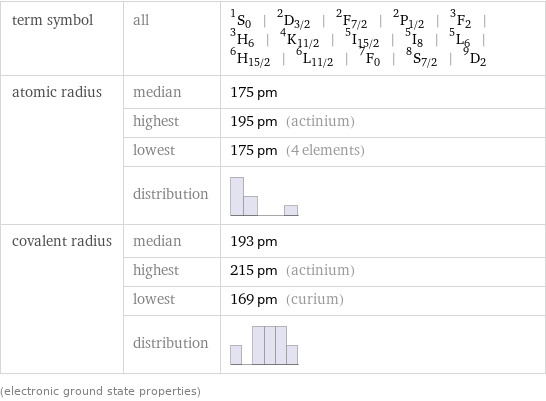
term symbol | all | ^1S_0 | ^2D_(3/2) | ^2F_(7/2) | ^2P_(1/2) | ^3F_2 | ^3H_6 | ^4K_(11/2) | ^5I_(15/2) | ^5I_8 | ^5L_6 | ^6H_(15/2) | ^6L_(11/2) | ^7F_0 | ^8S_(7/2) | ^9D_2 atomic radius | median | 175 pm | highest | 195 pm (actinium) | lowest | 175 pm (4 elements) | distribution | covalent radius | median | 193 pm | highest | 215 pm (actinium) | lowest | 169 pm (curium) | distribution | (electronic ground state properties)
Abundances
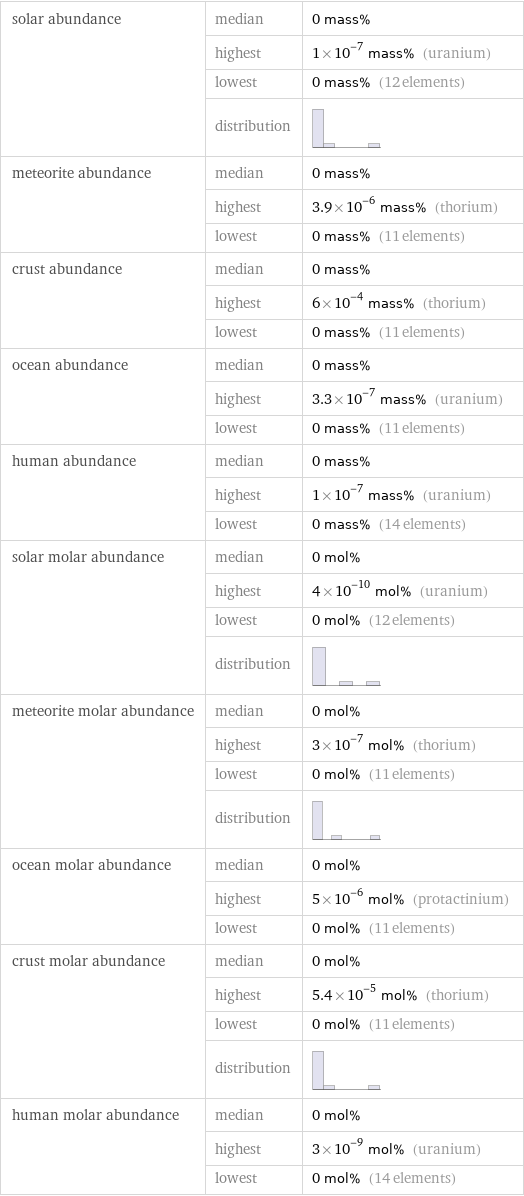
solar abundance | median | 0 mass% | highest | 1×10^-7 mass% (uranium) | lowest | 0 mass% (12 elements) | distribution | meteorite abundance | median | 0 mass% | highest | 3.9×10^-6 mass% (thorium) | lowest | 0 mass% (11 elements) crust abundance | median | 0 mass% | highest | 6×10^-4 mass% (thorium) | lowest | 0 mass% (11 elements) ocean abundance | median | 0 mass% | highest | 3.3×10^-7 mass% (uranium) | lowest | 0 mass% (11 elements) human abundance | median | 0 mass% | highest | 1×10^-7 mass% (uranium) | lowest | 0 mass% (14 elements) solar molar abundance | median | 0 mol% | highest | 4×10^-10 mol% (uranium) | lowest | 0 mol% (12 elements) | distribution | meteorite molar abundance | median | 0 mol% | highest | 3×10^-7 mol% (thorium) | lowest | 0 mol% (11 elements) | distribution | ocean molar abundance | median | 0 mol% | highest | 5×10^-6 mol% (protactinium) | lowest | 0 mol% (11 elements) crust molar abundance | median | 0 mol% | highest | 5.4×10^-5 mol% (thorium) | lowest | 0 mol% (11 elements) | distribution | human molar abundance | median | 0 mol% | highest | 3×10^-9 mol% (uranium) | lowest | 0 mol% (14 elements)