Input interpretation
iron(II) chromite | strontium cyanide dihydrate
Chemical names and formulas
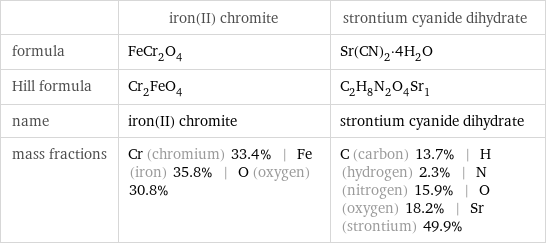
| iron(II) chromite | strontium cyanide dihydrate formula | FeCr_2O_4 | Sr(CN)_2·4H_2O Hill formula | Cr_2FeO_4 | C_2H_8N_2O_4Sr_1 name | iron(II) chromite | strontium cyanide dihydrate mass fractions | Cr (chromium) 33.4% | Fe (iron) 35.8% | O (oxygen) 30.8% | C (carbon) 13.7% | H (hydrogen) 2.3% | N (nitrogen) 15.9% | O (oxygen) 18.2% | Sr (strontium) 49.9%
Structure diagrams
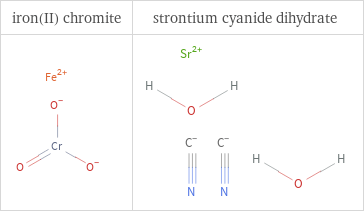
Structure diagrams
Basic properties
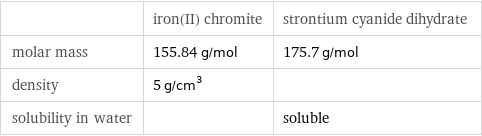
| iron(II) chromite | strontium cyanide dihydrate molar mass | 155.84 g/mol | 175.7 g/mol density | 5 g/cm^3 | solubility in water | | soluble
Units
